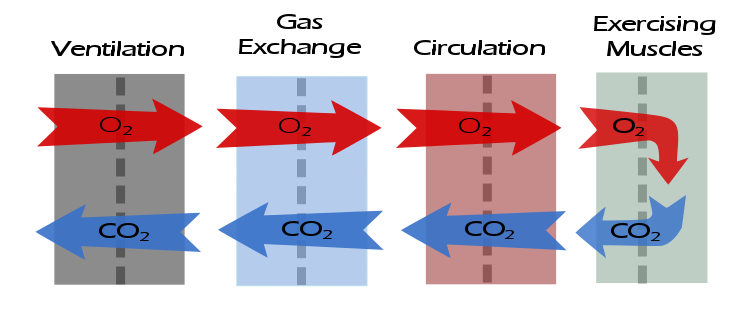
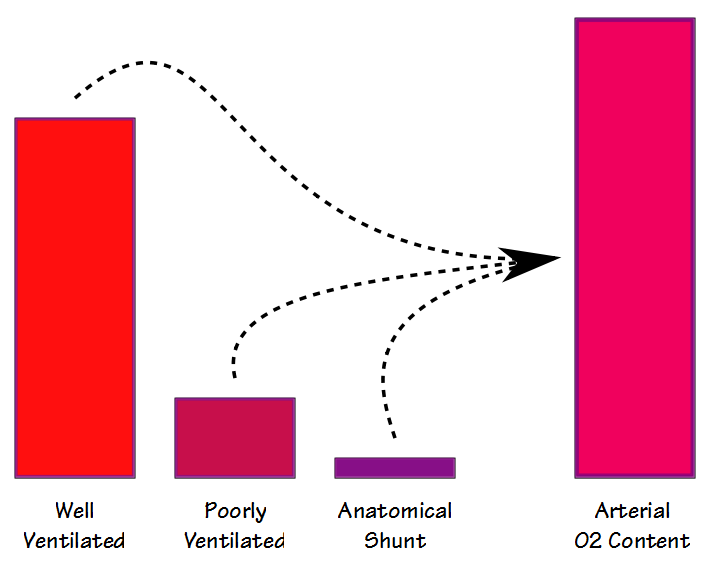
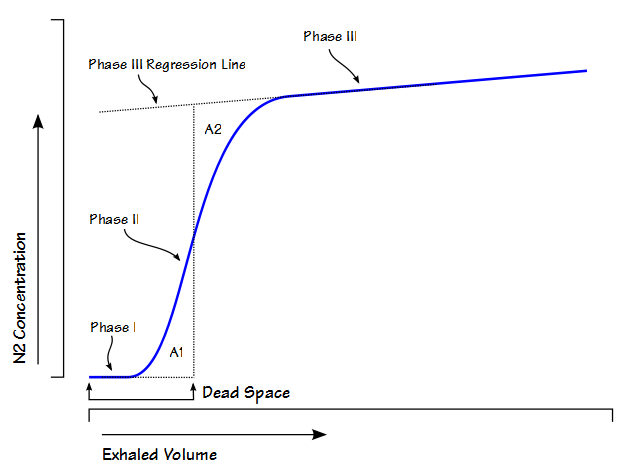
After having gone through the descriptive checklists for ventilatory, gas exchange and circulatory limitations the reason(s) for a patient’s exercise limitation, if any, should be reasonably clear. However, one of the first questions that should be asked when reading an exercise test is what was the purpose of the test?
- Maximum safe exercise capacity for Pulmonary Rehab?
- Rule in/rule out exercise-induced bronchospasm?
- Pre-operative assessment?
- Dyspnea of uncertain etiology?
- What is the primary limitation to exercise (pulmonary or cardiac)?
- Is deconditioning suspected?
The interpretation and summary should address these concerns.
The descriptions checklist is the main groundwork for the actual interpretation and any abnormal findings there may signal the need for specific comments. The interpretation should start by indicating whether or not the patient’s exercise capacity was normal and then should indicate the presence or absence of any limitations.
What was the patient’s maximum exercise capacity (maximum VO2)?
- >120% = Elevated
- 80% to 120% = Normal
- 60% to 79% = Mildly reduced
- 40% to 59% = Moderately reduced
- <40% = Severely reduced
Example: There was a {elevated | normal | mildly reduced | moderately reduced | severely reduced} exercise capacity as indicated by the maximum oxygen consumption of XX%.
Continue reading