I was reading an article recently that made an off-hand reference to the 100% oxygen shunt fraction test. Results from the test were included in the data analysis but the equations the researchers used were not presented nor were they referenced, nor was the procedure described. This is probably because the shunt fraction test and its equations are very much old-school pulmonary physiology but even if the subject is probably covered at one time or another in physiology classes I suspect that some of the issues involved in the calculation are not as well understood as they should be.
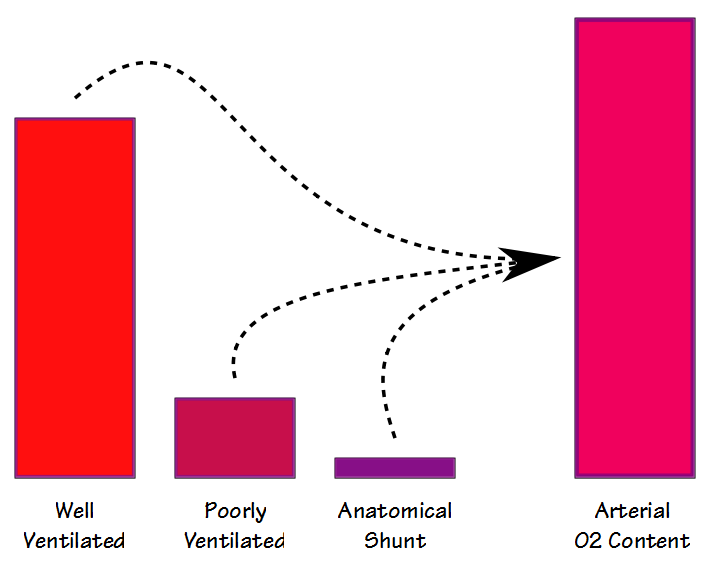
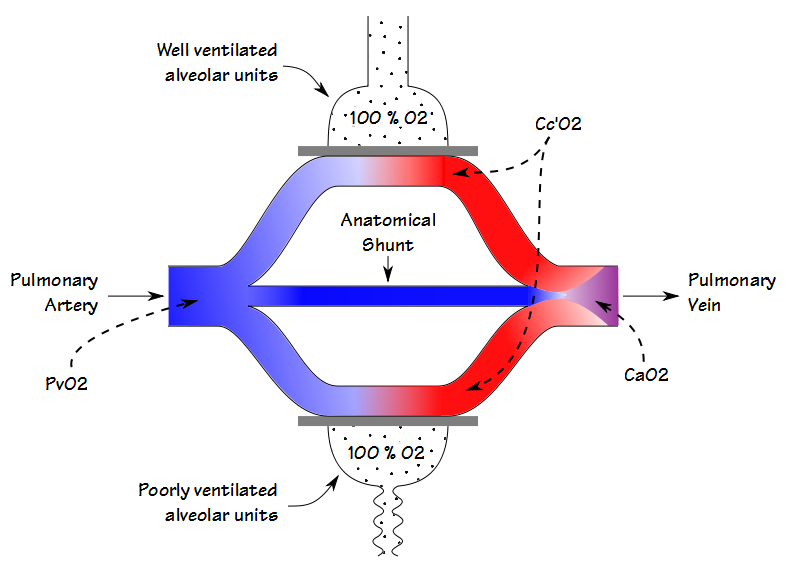
There are some similarities between the deadspace-tidal volume ratio (Vd/Vt) and the shunt fraction but even though they are both are involved in gas exchange (and to some extent they also correlate with each other) they are measuring different things. When blood flows through the lung some blood passes through well ventilated alveoli and becomes fully saturated; some blood passes through poorly ventilated alveoli and is only partially saturated; and some bypasses the alveoli entirely. The resulting arterial oxygen content is the summed average of all of these compartments.
There are two different ways that shunt fraction can be measured and calculated; physiological and anatomical. The physiological shunt equation can be performed at any FiO2 (but usually around the FiO2 of room air) and requires that arterial and mixed venous blood samples be taken more or less simultaneously and then analyzed for PO2 and SaO2. The basic formula is:
Where:
Qs = blood flow through shunt
Qt = total blood flow
Cc’O2 = pulmonary capillary O2 content
CaO2 = arterial O2 content
CvO2 = mixed-venous O2 content
Oxygen content is the milliliters of oxygen per liter of blood and is calculated from:
Where:
Hb = hemoglobin (grams/decaliter)
SO2 = oxygen saturation (%)
PO2 = oxygen partial pressure
The pulmonary capillary O2 content cannot be measured directly (and strictly speaking it is more of a conceptual value than a real one) and is usually estimated from the alveolar air equation (although “ideal” pulmonary capillary blood has a PO2 gradient of about 1 mm Hg from alveolar air this is insignificant enough that it is usually ignored).
Where:
Pb = barometric pressure in mm Hg
FiO2 = fractional concentration of inspired oxygen
PaCO2 = arterial partial pressure of CO2
RER = respiratory exchange ratio
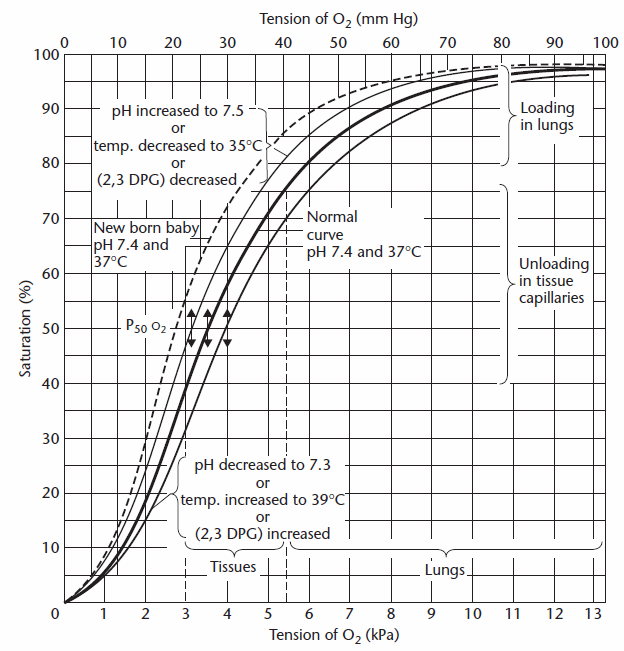
The oxygen content of the pulmonary capillaries is determined by first estimating the oxygen saturation from the PAO2 and this can be done either visually from the oxygen dissociation curve:
or from Severinghaus’s formula:
and then calculating Cc’O2 accordingly.
Note: Interestingly neither the oxygen dissociation curve nor Severinghaus’s formula take carboxyhemoglobin (or methemoglobin) into account. For that matter, this issue has not been included in any of the textbook discussions of shunt fraction I’ve read. COHb skews the relationship between PO2 and SO2 (downwards if you’re working from PO2 to SO2, upwards if you’re working from SO2 to PO2). Normal COHb levels in non-smokers are 1-2 and this amount of COHb is unlikely to make a significant difference in the shunt fraction calculations. In the absence of any firm guidelines however, when higher levels of COHb are present they should probably be used to adjust Cc’O2 accordingly.
Taking normal values and working backwards, PAO2 is:
The pulmonary capillary oxygen saturation is therefore:
And the pulmonary capillary oxygen content is:
Mixed venous blood nominally has a PO2 of 40 and an oxygen saturation of 75%, so:
CaO2 will then be calculated from an individual’s actual PaO2 and SaO2. Depending on the specific results the shunt fraction will be:
The physiological shunt fraction can only be calculated when both the arterial and the mixed-venous PO2 and SO2 are known. For this reason it is most often performed in a cardiac cath lab, operating room or intensive care unit where indwelling arterial and central venous lines are relatively common. The physiological shunt calculation cannot differentiate between the shunting caused by poorly ventilated alveolar units and that from an anatomical shunt, however. The anatomical shunt fraction can be calculated by a separate procedure however, and this is where the 100% O2 test comes into play.
By having a patient breath 100% O2 until the nitrogen has been washed out of their lung (nominally 20 minutes), the oxygen concentration in even poorly ventilated units will approach 100%. This means that the partial pressure and saturation of blood leaving both the poorly and the well ventilated alveolar units will be the same. For this reason, any decrease in the arterial oxygen content will be due solely to an anatomical shunt.
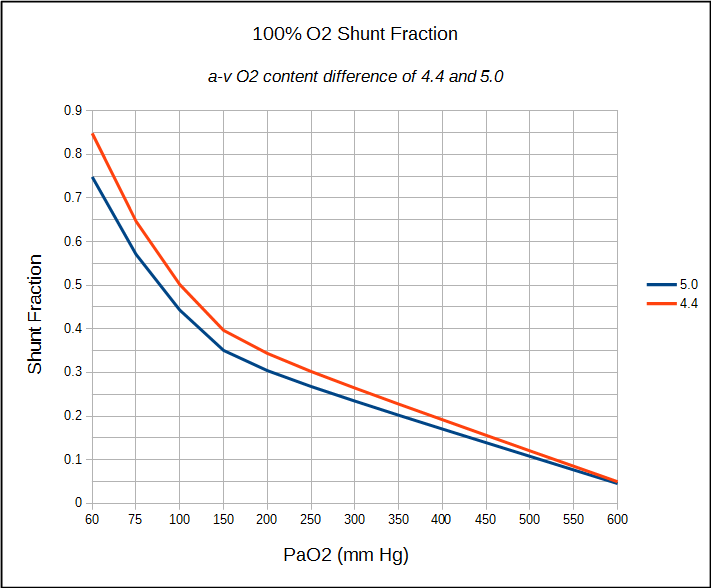
If a patient has an indwelling central venous catheter, the calculation of anatomical shunt can proceed the same way as already detailed. If only an arterial sample can be obtained (which is usually the case in a PFT Lab) an arterial-venous O2 content difference of between 4.4 and 5.0 can be assumed and the shunt fraction calculated accordingly.
The limitations of the shunt fraction calculations have to do in part with some of the assumptions about normal values and in part with the accuracy of blood gas measurements. The alveolar air equation, for example, assumes that the respiratory exchange ration (RER) is 0.8 but the only way to be sure is by actually measuring VO2 and VCO2. Strictly speaking, an RER that is different than 0.8 will probably not make a significant difference in calculated PAO2, Sc’O2 and Cc’O2, but it is still an assumption. Using an a-v O2 content difference of 4.4 to 5.0 on the other hand, is a much larger assumption. It is justified to some extent by the fact that the 100% O2 test is usually made at rest and these are reasonable values for an individual at rest but again, it is an assumption.
Far more concerning are the limitations in accurately measuring PaO2 and SaO2, particularly at higher FiO2’s. Two different studies have shown that the type of syringe used to obtain an ABG (glass versus plastic) and how it was stored (on ice or at room temperature) made a significant difference in the calculated shunt fraction even when the ABG samples were analyzed quickly. When there was a longer wait before analysis the error in PaO2 could cause the calculated shunt fraction to be twice as large as it really was. The reason this happens is partly due to diffusion through the plastic syringes and partily to continued metabolism within a blood sample when kept at room temperature. The least amount of change was seen when glass syringes kept on ice.
Interestingly, a similar study with ABG samples taken at normal FiO2 (PO2 ≈ 100) showed the opposite effect. The measured PO2 tended to rise, again more in plastic syringes than in glass, and again this likely due to diffusion. Interestingly, PO2 fell in glass syringes kept on ice and the authors, Knowles et al, point out that the solubility of O2 rises as temperature falls and that with more O2 in solution PO2 may decrease.
Finally, blood gas analyzers are usually calibrated using gas concentrations in the normal physiological range. Any arterial blood sample with PO2 above 200 mm Hg is well outside this range and I am concerned about what kind of error bar there is for PO2’s that are even higher. Pretto et al used blood tonometered with 95% O2 and 5% CO2 but interestingly they did not report the measured PO2 but only the change in PO2 over time. Smeenk et al obtained blood samples from individuals undergoing the 100% oxygen test as a pre-op assessment for coronary bypass surgery and the average PO2 of their gold standard samples (glass syringe, iced, 5 minute delay) was 590 mm Hg. This is an A-a gradient of around 80 mm Hg and may well be appropriate, but it also means that the average anatomical shunt fraction was 10% and Cotes et al indicates that the normal anatomical shunt for individuals in the same age range is around 4%.
The shunt fraction test is not commonly performed in pulmonary function labs. True anatomic shunts are relatively rare and the most appropriate patient for the 100% O2 shunt fraction test would be one with a reduced SaO2 at rest that does not significantly improve with supplemental O2.
The physiological shunt fraction could be considered the reverse side of Vd/Vt. Perfusion inhomogeneities exist just as much as ventilation inhomogeneities but this may be overlooked because pulmonary function testing is oriented far more around the ventilation side of respiration than the perfusion side. Ventilation and perfusion inhomogeneities are core features of many pulmonary diseases. For this reason the shunt fraction and the differences between its physiological and anatomical components need to be part of the education of all pulmonary technologists. Like Vd/Vt however, there are also limitations to the accuracy of the shunt fraction calculation both from assumptions that may or may not be reasonable, and from the measurement accuracy of PO2 and SO2.
References:
Aboab J, Louis B, Jonson B, Brochard L. Relation between PaO2/FiO2 and FiO2: a mathematical description. Chapter in Applied Physiology in Intensive Care Medicine, Pinsky MR, Brochard L, Mancebo JM editors. Springer-Verlag Heidelberg, 2006.
Conrad SA, Kinasewitz GT, George RB. Pulmonary Function Testing. Principles and Practice. Churchill Livingston Publishing, 1984.
Cotes JE, Chinn DJ, Miller MR. Lung Function, 6th Edition. Blackwell Publishing, 2006.
Knowles TP, Mullin RA, Hunter JA, Douce FH. Effects of syringe material, sample storage time and temperature on blood gases and oxygen saturation in arterialized human blood samples. Respir Care 2006; 51(7): 732-736.
Pretto JJ, Rochford PD. Effects of sample storage time, temperature and syringe type on blood gas tensions in samples with high oxygen pressures. Thorax 1994; 49: 610-612.
Smeenk FWJM, Janssen JDJ, Arends BJ, Harff GA, van den Bosch JA, Schonberger JPAM, Postmus PE. Effects of four different methods on sampling arterial blood and storage time on gas tensions and shunt calculation in the 100% oxygen test. Eur Respir J 1997; 10: 910-913.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License


Great posting. When I started out 30 years ago in PFTs 100% O2 shunt studies were popular. They have over the years become less so, but now I am being asked to do these studies more regularly.
I have a question – historically we have assumed that if the resting A-a gradient is abnormal (high) then the calculation of 100% O2 shunt fraction is not accurate, presumably due to the V/Q issues at parenchymal level. However, if we confirm that we have complete nitrogen washout from breathing 100% O2 then the abnormal resting A-a gradient should not affect the shunt fraction calculation, especially given the number of assumptions we make in the calculation. Do you have a comment on this thought?
David –
Strictly speaking when you calculate shunt fraction using the 100% O2 technique an elevated A-a gradient that is due to a gas exchange defect doesn’t matter. And, strictly speaking, if you performed a shunt fraction test while on room air and have measured arterial and mixed-venous O2 content it doesn’t matter either. As you say though, we usually make a number of assumptions when performing this kind of test, most often by assuming a “normal” a-v O2 content difference, and this alone puts a limit on the accuracy of the measurement.
– Richard
On a simple 30 minute shunt study done with mask and 100% FIO2. My Pao2 was 272 mmHg. The test results showed FIo2 at 1. I also had a ABG test done at room air. both were done at sea level. I am checking with the lab to see what confidence they have that the 100% value was obtained . Is it possible to have a 100% fio2 in such a test. If so my result puts me into the ALI category. My room air result was low 72mmHG calculated gradient 25mmHG room. I am curious as to experience labs with this test. Depending on the true value of the FIo2 . Unfortunately they did not take any pressures to determine venuous admixture during L/R heart cath. My calculations using your info puts me at 0.2 shunt. Heart is ruled out. Could you comment regarding FI02 @100% is it possible using a rebreather mask ?
Greg –
Whether or not you can get 100% O2 depends on the oxygen flow rate to the rebreather mask. Masks like that don’t seal all that well, and really aren’t meant to. If I was using a mask for a 100% O2 shunt study (I prefer using a noseclip, 2-way mouthpiece and a reservoir bag although patients say it gets uncomfortable after a while) I’d set a flow rate of at least 12-15 LPM in order to ensure the patient was receiving something close to 100% FiO2. To be honest, it’s been at least 10 years since I last did a shunt study and even at that we never did them all that often.
Regards, Richard
I called the lab. They used a re-breather mask/bag and assured me that I was getting 100% o2 for a ll practical purpose. So after searching literature. I see no way this could NOT be an intrinsic shunt (now V/Q). I am looking for a reliable pulmonary doctor to repeat. Any referrals ?
Greg –
Given that your A-a gradient on room air was 25 mmHg I’m inclined to agree that the results from your 100% O2 shunt test are reasonably accurate. The possible reasons for an apparent shunt are many and you’d certainly need more tests (V/Q scan of course, DLCO too) to be able to pinpoint the reasons. If it was me I’d look for a pulmonary physician that specializes in pulmonary hypertension (not because I think you necessarily have pulmonary hypertension but because they should be familiar with your type of problem) at an academic/teaching hospital.
Regards, Richard
Is the normal shunt fraction 10% or less.
Paul –
Depends on which reference you want to use, a normal shunt fraction is between 4% and 10%.
Regards, Richard