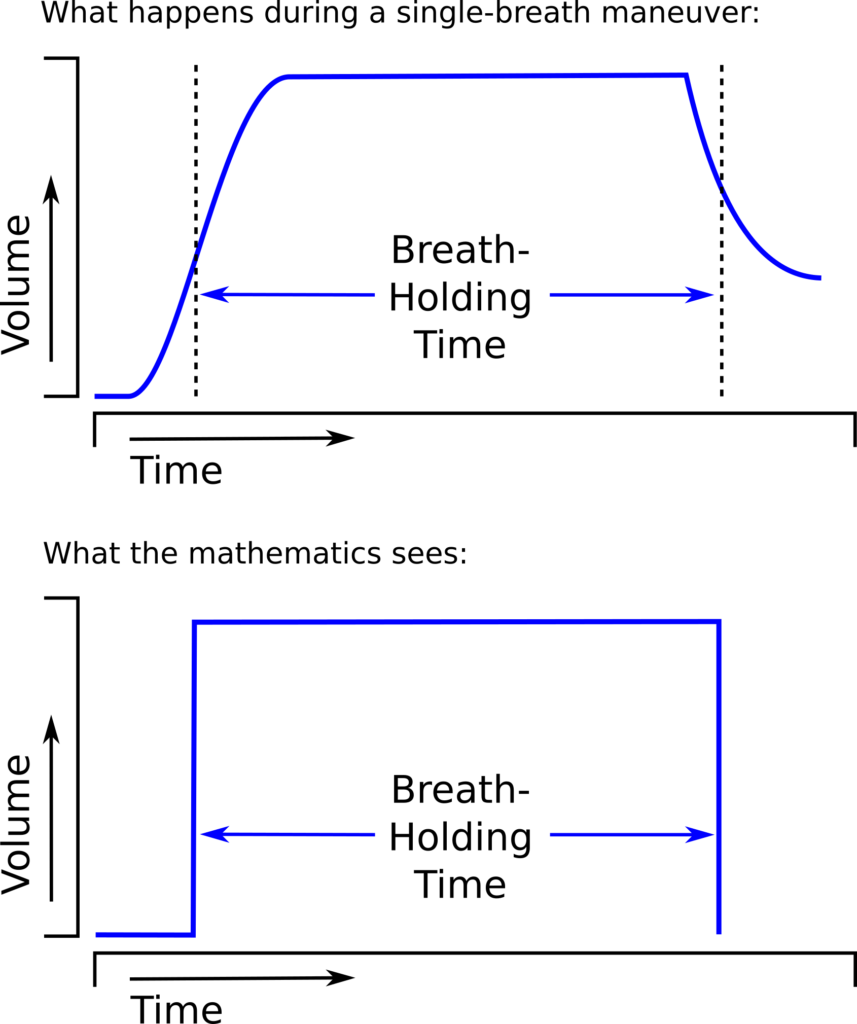
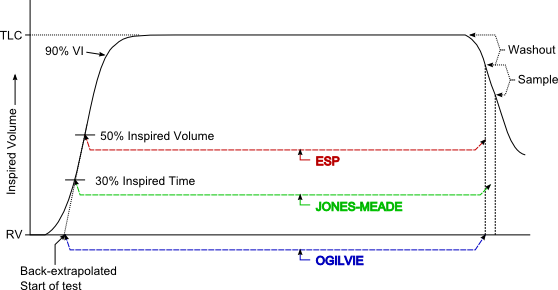
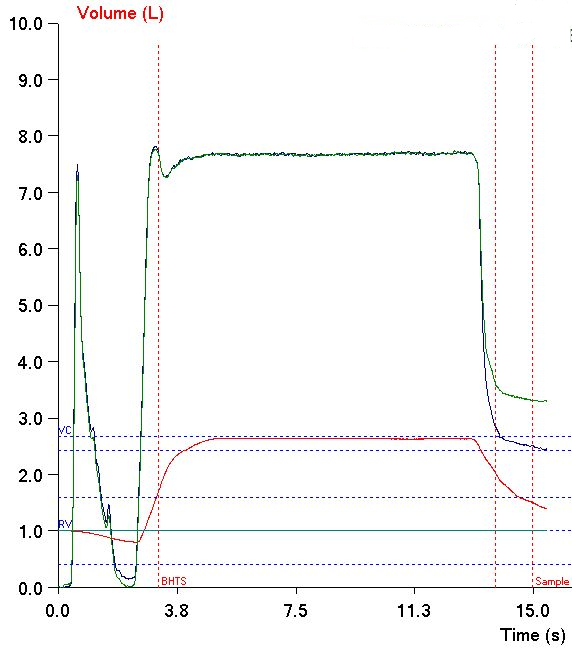
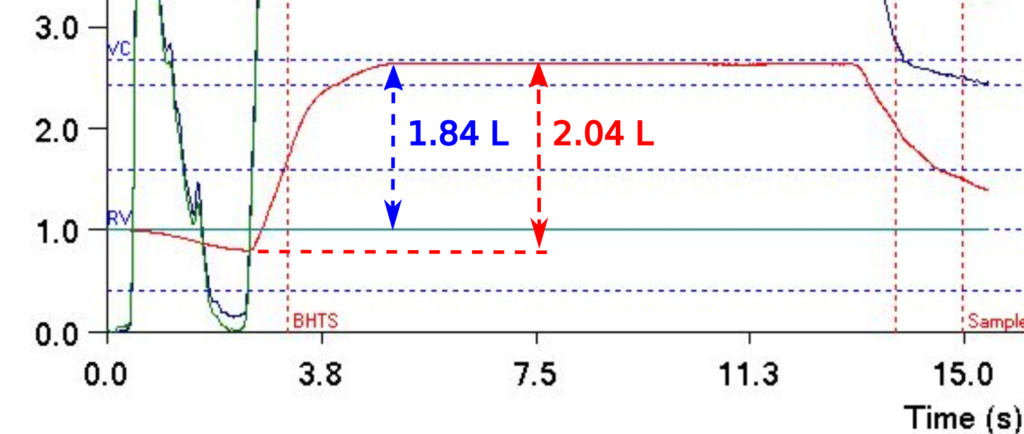
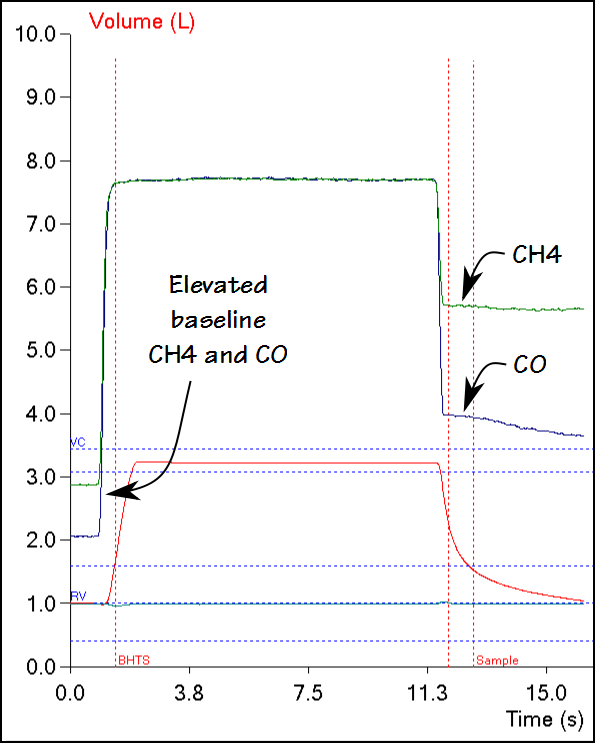
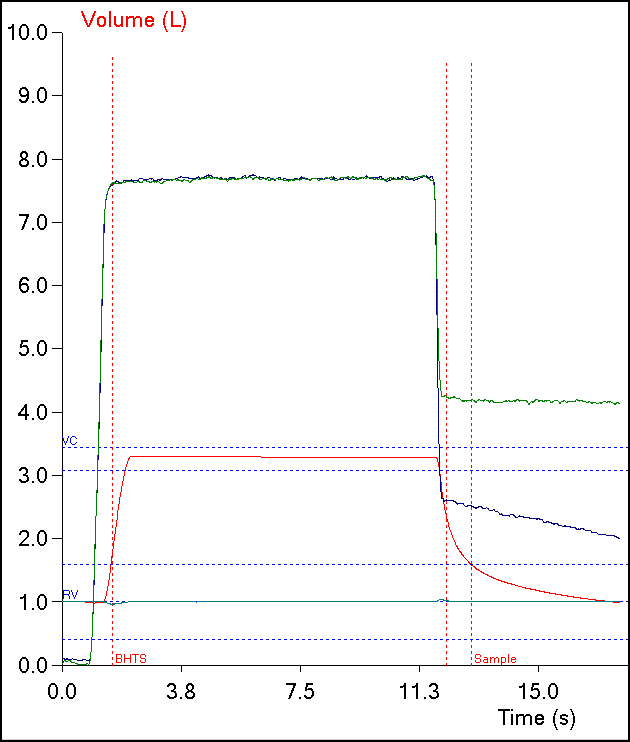
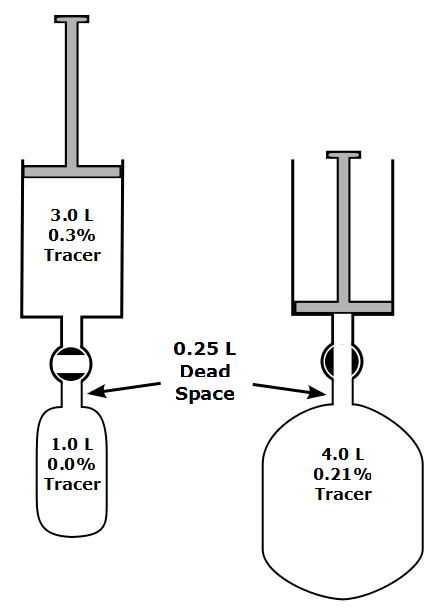
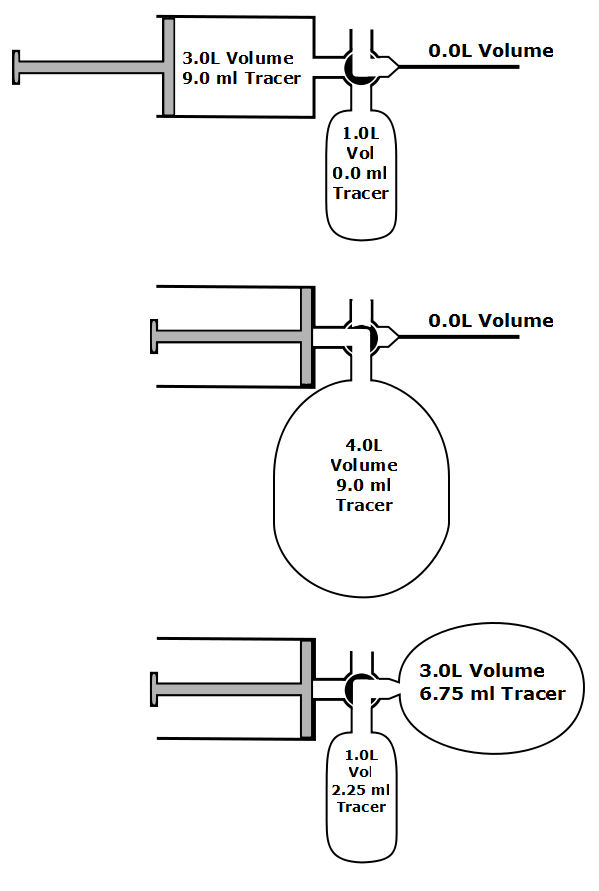
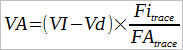One of the limitations of the single-breath DLCO is that the equation used to calculate results implicitly assumes that the entire breath-holding period occurs at TLC. Mathematically, what happens to the diffusion of carbon monoxide (CO) during inspiration and expiration is not a consideration:
The different approaches towards measuring breath-holding time (BHT) make allowances for inspiration and expiration to one extent or another but realistically they should be considered fudge factors.
The 3-equation DLCO was first proposed by Graham et al in 1980 and it received its name because there is a separate equation for each phase of the single-breath DLCO maneuver. The individual equations are based on the mass-balance equation and attempt to account for the mass of CO inhaled, absorbed and exhaled during the single-breath maneuver. One of the most significant differences is that an iterative approach is used to determine DLCO. Specifically, an initial estimate of DLCO is made and then compared against the values measured during the three phases. Any differences in observed versus expected values is used to re-estimate the DLCO, and then re-compare it. The authors indicated that 10 iterations are usually sufficient to converge on a DLCO value that meets all measured conditions with a high degree of accuracy.