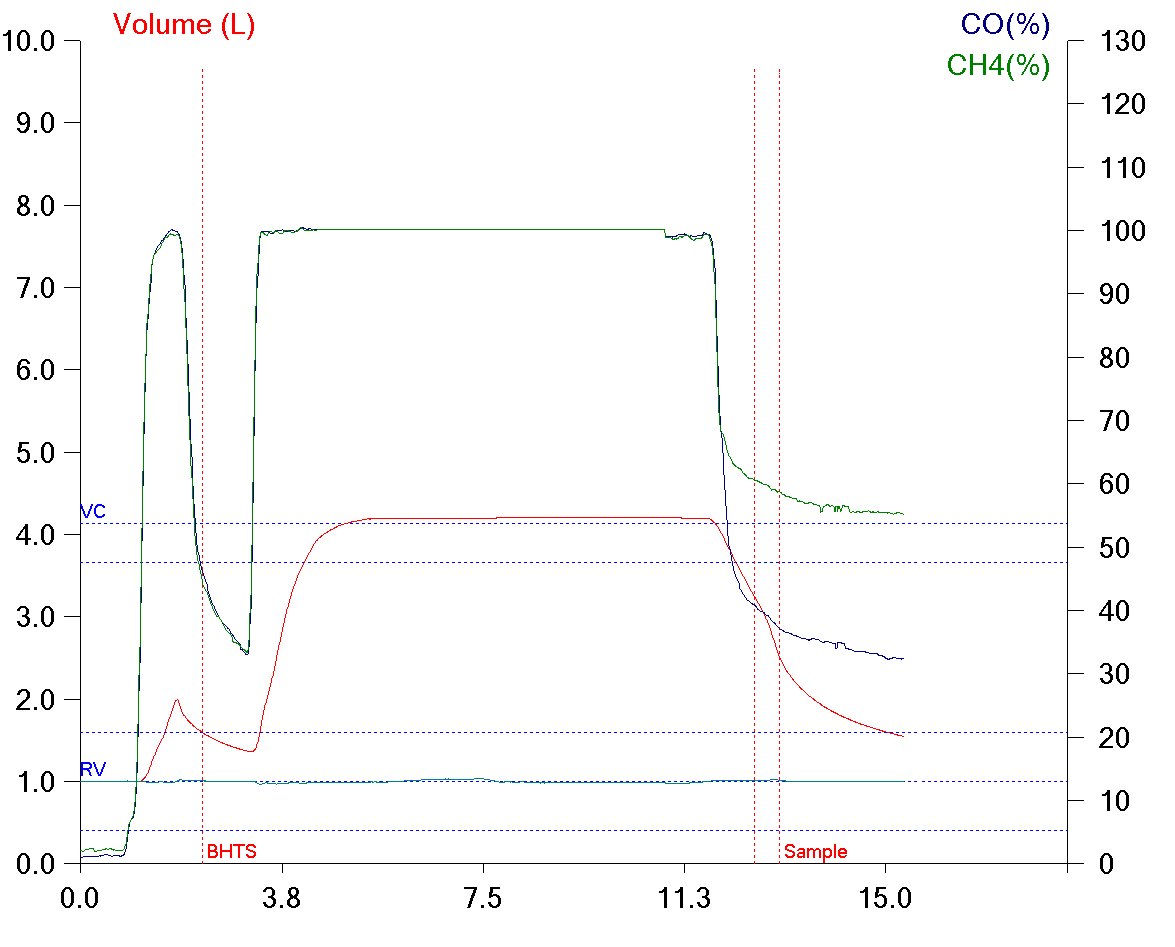
The new ERS/ATS standards for DLCO testing were published in the January issue of the European Respiratory Journal. The article was published as open access and can be downloaded from the ERJ website.
The biggest difference between the new standards and those from 2005 is that they are now primarily oriented towards Rapid-response Gas Analyzers (RGA). The authors explicitly state that the new standards do not make older systems that use discrete alveolar sampling and slower gas analyzers obsolete, but many of the new suggestions and requirements for labs and manufacturers require systems with a RGA.
The differences between the 2017 and 2005 standards that I’ve been able to find include:
♦ Flow accuracy was not specified in the 2005 standard but is now required to be ± 2% over a range of ± 10 L/sec.
♦ Volume accuracy is now required to be ± 2.5% (± 75 ml) instead of ± 3.5%. Notably the 2005 standard included a ± 0.5% error in the calibrating syringe. The accuracy of the 3-liter syringe is now stated separately. In the 2005 standard volume accuracy was over an 8-liter range. No volume range is specified in the 2017 standard.
♦ RGA response time (analyzer rise time) had not previously been specified but is now required to be ≤150 milliseconds. Sample transit time was discussed but no specific recommendations were made. Sample transport issues such as Taylor dispersion, gas viscosity and turbulence at gas fittings was also discussed and although it was suggested that manufacturers attempt to minimize these effects no specific recommendations were made.
♦ Analyzer linearity for both RGA and discrete sample systems has been relaxed to ± 1.0% in the 2017 standards from ± 0.5% in the 2005 standards.
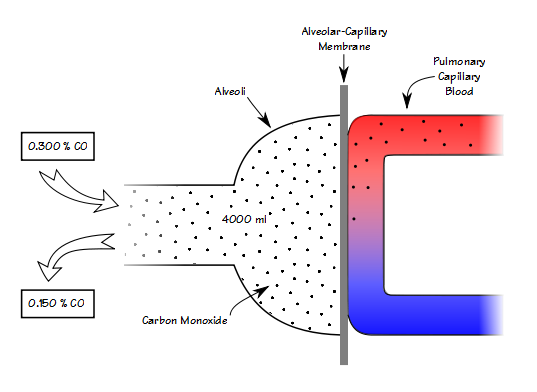
♦ CO analyzer accuracy for both RGA and discrete sample systems is now specified as ≤10 ppm (which is ±0.3% of 0.3% CO). It was previously specified as ± 0.0015% (which is ± 0.5% of 0.3% CO).
♦ Interference from CO2 and water vapor for both RGA and discrete sample systems is now specified as ≤10 ppm error in CO (when CO2 and water vapor are ≤5%). Interference was recognized as a problem in the 2005 standard but error limits were not specified.
♦ Digital sampling rate was not discussed or specified in the 2005 standards. It is now specified as a minimum of ≥100 hz with a resolution of 14 bits. A 1000 hz sampling rate is recommended.
Continue reading