In patients with lung disease the use of supplemental oxygen during exercise increases oxygen consumption, endurance time and maximum workload, and decreases the sensation of dyspnea without increasing minute ventilation and maximum heart rate. My lab is occasionally asked to perform a CPET with an elevated FIO2 (hyperoxic CPET). We are capable of doing this but I’ve always had reservations, partly about the logistics involved in performing the CPET but more importantly with the interpretation of the results.
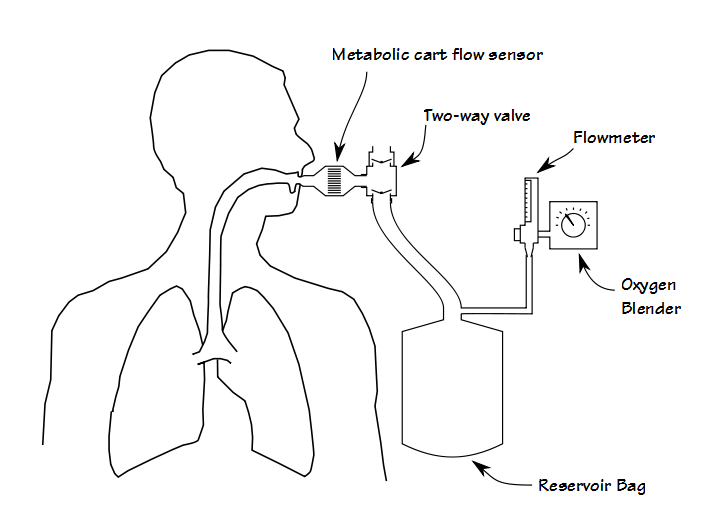
First, although it is certainly possible to perform some kinds of exercise test while the patient gets oxygen via a nasal cannula or mask, adding oxygen during a CPET requires that the patient breathes a hyperoxic gas mixture through their mouthpiece. Most commonly this is done by adding a two-way valve to the test system that is in turn attached to a reservoir bag which is filled from an oxygen blender.
Although functional, this adds extra dead-space and the valves add extra resistance, both of which increases the patient’s work of breathing. From a practical standpoint it also adds a fair amount of extra weight to the breathing manifold, often more than is comfortable for the patient. This means that some method for supporting the manifold must also put in place. About 25 years ago I performed CPETs using a treadmill that had a support arm and at that time the approach recommended by the equipment manufacturer was to suspend the breathing manifold using rubber tubing. This worked in that it supported the weight of the breathing manifold, but it didn’t do anything about its mass or inertia and when a patient transitioned from a walk to a jog, the mouthpiece manifold would bang against the patient’s teeth and mouth.