In patients with lung disease the use of supplemental oxygen during exercise increases oxygen consumption, endurance time and maximum workload, and decreases the sensation of dyspnea without increasing minute ventilation and maximum heart rate. My lab is occasionally asked to perform a CPET with an elevated FIO2 (hyperoxic CPET). We are capable of doing this but I’ve always had reservations, partly about the logistics involved in performing the CPET but more importantly with the interpretation of the results.
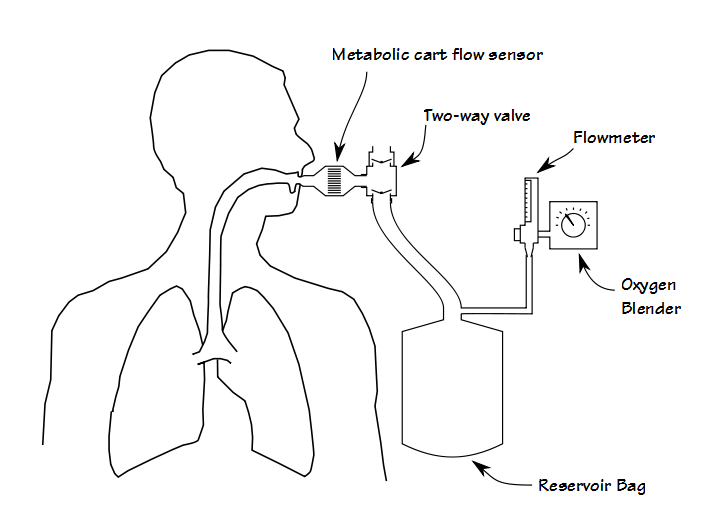
First, although it is certainly possible to perform some kinds of exercise test while the patient gets oxygen via a nasal cannula or mask, adding oxygen during a CPET requires that the patient breathes a hyperoxic gas mixture through their mouthpiece. Most commonly this is done by adding a two-way valve to the test system that is in turn attached to a reservoir bag which is filled from an oxygen blender.
Although functional, this adds extra dead-space and the valves add extra resistance, both of which increases the patient’s work of breathing. From a practical standpoint it also adds a fair amount of extra weight to the breathing manifold, often more than is comfortable for the patient. This means that some method for supporting the manifold must also put in place. About 25 years ago I performed CPETs using a treadmill that had a support arm and at that time the approach recommended by the equipment manufacturer was to suspend the breathing manifold using rubber tubing. This worked in that it supported the weight of the breathing manifold, but it didn’t do anything about its mass or inertia and when a patient transitioned from a walk to a jog, the mouthpiece manifold would bang against the patient’s teeth and mouth.
About 15 years ago my lab was moved from one location to another within the hospital and we weren’t able to bring the treadmill with the support arm with us. For this reason we started having one of our technicians stand next to the treadmill and help support the additional weight and found that this actually worked rather well. When we transitioned to a bicycle ergometer we continued to do this. This meant that we needed an additional technician to perform the CPET but they are only needed during the actual CPET.
Since the patient is breathing from a reservoir bag, it is important that this remains filled with a sufficient amount of gas. Although most patients that have a CPET with supplemental oxygen have lung disease, that doesn’t mean that they aren’t able to reach a relatively high minute volume. This in turn means that both the oxygen blender and the flowmeter must be able to provide sufficiently high gas flow rates during the test. It also means that care must be taken during the test to adjust the flow rate in order to keep the reservoir bag filled. If it isn’t you will limit the patient’s tidal volume and the test will end earlier than it should (which I know all too well from experience). We usually have the technician that is supporting the patient’s mouthpiece keep an eye on the reservoir bag volume and adjust the flow rate.
The logistics of providing a hyperoxic gas mixture will raise the complexity of a CPET, but not unmanageably so. A far bigger problem is interpreting the results although to some extent this depends on the reason the CPET was being performed in the first place.
Numerous studies have shown that patients with COPD and ILD will improve their maximum VO2 when given supplemental oxygen and that this occurs without increasing their minute ventilation or heart rate. This also acts to skew other gas exchange values. About 15 years ago I was reviewing an exercise test performed with an FIO2 of 35% on a patient with moderate-severe COPD. I noticed that the patient’s Ve/VCO2 at anaerobic threshold was normal (<35), the SpO2 was above 95% throughout the test and that the maximum PETCO2 was above 40. These factors would usually indicate that the patient had no pulmonary vascular disease, but the patient had performed a DLCO test about a week previously and it was around 40% of predicted.
My best guess as to what was happening was that by lowering the minute ventilation needed for a certain level of VO2, that also means that it was lowered for VCO2 as well, which in turn means that the Ve/VCO2 was lowered. Normally PETCO2 on patients with COPD is reduced because of an increased Vd/Vt, but in this case I suspect that the patient was hypoventilating relative to his VCO2 which in turn would cause PETCO2 to rise. The elevated PETCO2 could also be because the patient was able to reach a relatively high level of exercise and when this occurs PETCO2 can be affected by the increased PvCO2 returning to the lung. Some studies have showed that PaCO2 increases with an elevated FIO2 so the first reason may be more correct.
Since that time I’ve paid careful attention to the results from hyperoxic CPETs but haven’t seen as distinct a shift in gas exchange values. Although I suspect this occurs to one degree or another when an elevated FIO2 is used the magnitude of the effect is difficult to assess on a case by case basis. Changes in Ve/VCO2 have not been studied to any extent so it is unclear to what degree it changes, if it changes at all. Interestingly research has showed that VCO2 tends to decrease when an elevated FIO2 is used and this appears to be related to a reduced lactate.
The last several times we’ve performed CPETs with supplemental oxygen they were for the pre-op assessment of oxygen-dependent patients, and I think this is particularly problematic. One of the most critical results obtained from a CPET is the maximum oxygen consumption and this is used as part of an algorithm for determining the safety of a surgical procedure. A max VO2 above 15 ml/kg/min is generally taken as an indication that thoracic surgery is reasonably safe. This threshold comes from studies performed on room air however, and it is unlikely that a max VO2 greater than 15 ml/kg/min that was obtained with an elevated FIO2 has the same meaning.
In general, the maximum VO2, exercise time and workload increases when an elevated FIO2 is used during exercise. At the same time the maximum minute ventilation and maximum heart rate do not change. The VO2 at anaerobic threshold also tends to increase. There is some correlation between the FIO2 used during exercise and the degree to which VO2 improves but the degree of improvement is not predictable within a single individual. The problem that comes with interpreting a hyperoxic CPET is that the reference and threshold values are based on room air testing. Values that would be abnormal when a CPET was performed with room air are often within normal limits when a hyperoxic gas mixture is used. Unless there are very specific goals in performing a CPET with an elevated FIO2 interpretation of results is very difficult.
Given this fact it is unclear to me what clinical value a CPET with supplemental O2 really has. Having said that, since patients are able to exercise longer and reach a higher workload this gives an opportunity for cardiovascular limitations to arise that would otherwise wouldn’t be detected. An additional exception is when a CPET is performed prior to starting pulmonary rehabilitation when the rehabilitation is going to be performed with supplemental O2 since any limitations detected during the CPET are relevant to rehabilitation.
CPETs can be performed with an elevated FIO2. The methods for doing this are relatively straightforward but the interpretation of the results is made difficult by the fact that values that are routinely monitored during a CPET are significantly skewed by the use of an elevated FIO2 and the amount of skewing is not predictable. For this reason there are no clear guidelines for interpreting a hyperoxic CPET and that the reasons for performing such a test need to be clear before it is attempted.
References:
Astorino TA, Robergs RA. Effect of hyeroxia on maximal oxygen uptake, blood acid-base balance, and limitations to exercise tolerance. JEPOnline 2003; 6: 8-20.
Harris-Eze AO, Sridhar G, Clemens RE, Gallagher CG, Marciniuk DD. Oxygen improves maximal exercise performance in interstitial lung disease. Am J Respir Crit Care Med, 1994; 150: 1612-1622.
Marcus CL, Bader D, Stabile MW, Wang CI, Osher AB, Keens TTG. Supplemental oxygen and exercise performance in patients with cystic fibrosis with severe pulmonary disease. Chest 1992; 101: 52-57.
Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercuse
Stein DA, Bradley BL, Miller WC. Mechanisms of oxygen effects on exercise in patients with chronic obstructive pulmonary disease. Chest 1982; 81(1): 6-10.
Stellingwerff T, LeBlanc PJ, Hollidge MG, Heigenhauser GJF, Spriet LL. Hyperoxia decreases muscle glycogenolysis, lactate production and lactate efflux during steady-state exercise. Am J Physiol Endocrinol Metab 2006; 290: E1180-E1190.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License