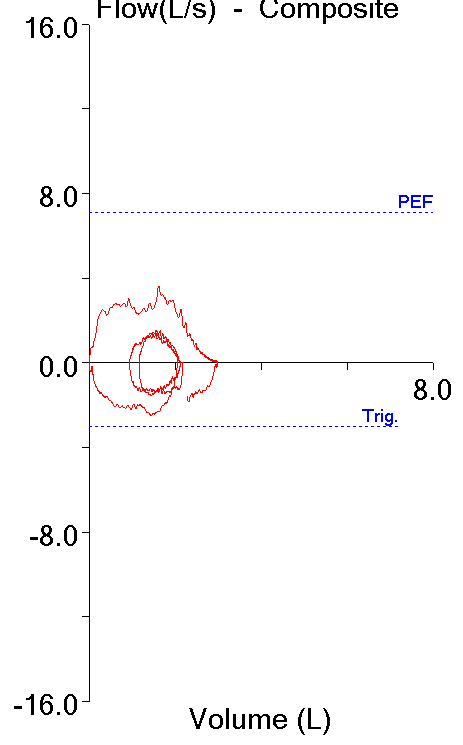
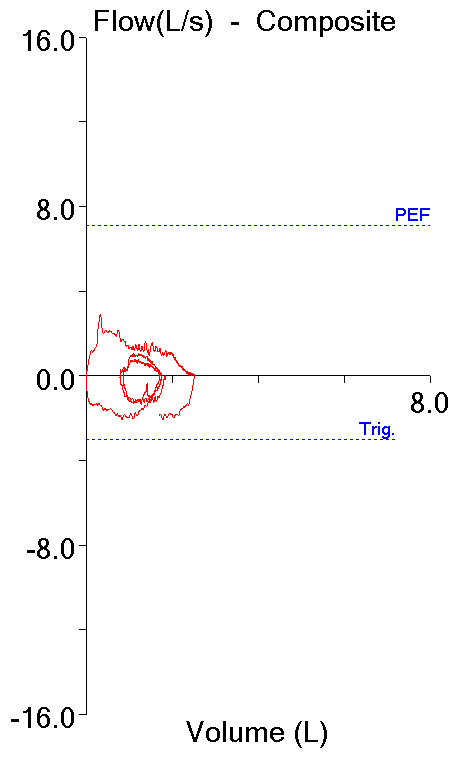
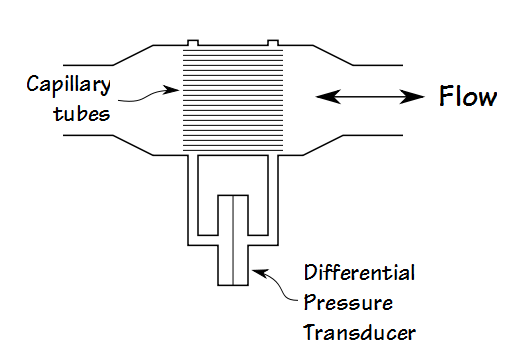
The Maximum Voluntary Ventilation (MVV) test was initially described in 1933. It was the first pulmonary function test that involved inspiratory and expiratory air flow in a significant way and for this reason it helped to set the stage both conceptually and technically for the FEV1, the FEV1/FVC ratio and our present understanding of obstructive lung diseases. MVV is reduced in a variety of conditions, including obstructive, restrictive and neuromuscular diseases, but a reduced MVV is non-specific and this limits its clinical utility. Nevertheless, it continues to be performed both in clinical labs and for research, and for this reason it would seem to be a good idea to know how to assess MVV results.
As usual, there are two aspects to assessing pulmonary function results; test performance and normal values.
Currently the ATS/ERS statement on spirometry contains the only available standard for performing the MVV test. Unfortunately this standard also contains some significant flaws. Its primary recommendation is that the MVV test be performed with a tidal volume that is approximately 50% of the forced vital capacity and a breathing frequency of around 90 breaths per minute. These recommended values are problematic and some simple mathematics will show why.
A respiratory rate of 90 BPM means that there is 2/3 of a second for both inhalation and exhalation. With a 1:1 ratio for inspiration and expiration, there is only 1/3 of a second for exhalation. Since it normally takes a full second to exhale approximately 75% of the vital capacity (i.e. the FEV1), 1/3 of a second would only allow time to exhale 25% of the vital capacity (not exactly true of course, but it helps prove the point). How then is it possible to exhale 50% of the vital capacity, twice that amount, in the same amount of time? The answer is that it isn’t and if it was somehow possible for somebody to actually meet the ATS/ERS recommended values they would have an MVV that would be 45% to 100% higher than any of the predicted MVV’s. I suspect the ATS/ERS agrees this is a problem since following the initial recommendation it also says that “… since there are little data on MVV acceptability criteria, no specific breathing frequency or volume is required”.
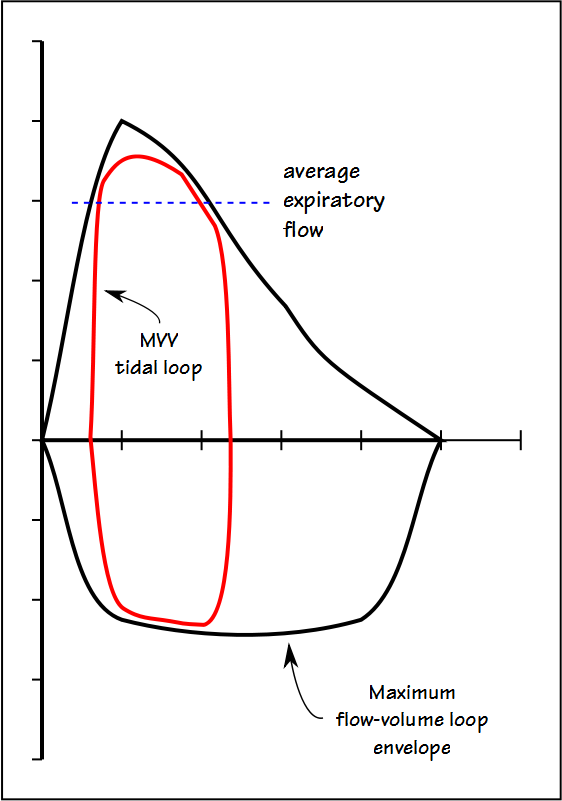
The fact is that no single tidal volume recommendation is going to work for all patients and this is because the MVV tidal volume has to reside mostly within the maximal flow-volume loop envelope.