Once again my lab was questioned by a research study’s primary investigator and study coordinator about why our lung volume results came out significantly lower than another lab’s. In order to be part of this study a subject has to have an RV that is greater than 150% of predicted. The RV we had obtained on a subject referred to the study was over a liter less than the results they had brought with them from another lab and for this reason the patient no longer qualified.
When I reviewed the subject’s test data from my lab it was clear to me that our test quality was good and more than met the ATS/ERS reproducibility criteria. We were given a copy of the subject’s report from the other lab and at first glance, the results look very typical for emphysema. Specifically the report showed very severe airway obstruction, a normal TLC, an elevated FRC and RV consistent with hyperinflation and a severely reduced DLCO. Our results however, showed a mixed defect with severe obstruction and a mildly reduced TLC.
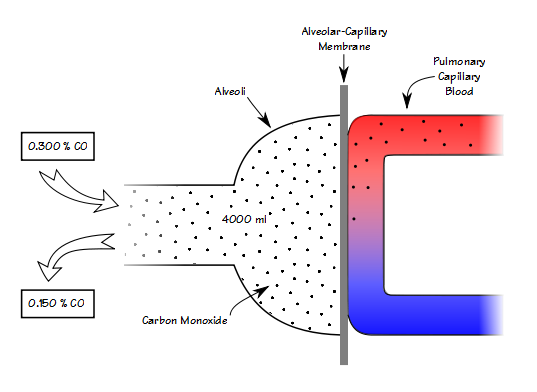
Getting accurate lung volume measurements is hard. Regardless of which measurement technique you use, in most instances any errors tend to cause lung volumes to be overestimated. When very severe airway obstruction is present unless you are careful about panting frequency, plethysmography will often overestimate FRC and TLC, and that may be what happened in this case.
But this isn’t about test quality or the reasons why I believe my lab is better than most others. Although the report was from a nearby hospital with a reputation for the quality of its patient care, when I started reviewing it I immediately started to see math errors among the predicted values. I’ve run across these kind of errors before but this report was from a different equipment manufacturer than last time and this means that these kind of errors are probably far more common than I ever would have expected.