I was reviewing PFT reports today and noticed that a patient appeared to have had the wrong gender entered in their demographic information. Specifically, the patient had an unambiguously masculine name but had been entered as female. Just to be sure I checked the patient’s on-line medical record and there he was listed as male. I had noticed from the trend report that the patient had been in the PFT lab numerous times. Since the basic patient demographics (name, date of birth, height, gender, etc.) are automatically forwarded into a new demographics record when a new PFT lab visit is created it struck me as odd that after all this time we had somehow managed to make a mistake with something as basic as gender. For this reason I thought it would be a good idea to see how far back this problem existed and started going back through the patient’s PFT records. About four visits ago the patient’s name suddenly changed to one that was unambiguously feminine.
I was immediately concerned that two different patient’s records had somehow gotten merged. The last time this happened was over 20 years ago and was due to an entry error in the patient ID that was further compounded by how the lab’s software handled new demographic records at the time. Merged records is therefore a symptom of a serious database problem but when I compared the date of birth of the two patients, I was immediately able to see that they were the same. Since this is incredibly unlikely my thought then was that the patient may have had a gender reassignment. When I went back to the patient’s online medical record and searched more carefully, I was able to find that this had occurred over a year ago. This is not the first time we’ve had a transgender patient and so it is an issue we’ve learned how to handle.
So what effect does gender reassignment have on an individual’s pulmonary function test results?
None whatsoever. Gender reassignment by itself does not affect FVC, FEV1, TLC or DLCO. What it does affect is how we interpret the test results and it can also cause some interesting data management problems that are worth noting.
All pulmonary function reference equations differentiate between genders. Although the differences between races and ethnicities is somewhat open to question, there is little doubt about the differences between genders. When individuals with the same height are compared, females universally have lower flow rates, volumes, respiratory muscle strength, gas exchange and oxygen consumption than males. Because lung function is determined during an individual’s childhood and adolescent developmental periods, gender reassignment does not affect lung function and when it is assessed this has to be done using reference equations that are appropriate to an individual’s original gender.
Depending on which way a gender reassignment occurs, results that would be considered normal for a female would likely look reduced for a male, and results that would be considered reduced for a male would likely look normal for a female. The selected gender will therefore make a difference about what an individual’s PFT results look like to a reviewer.
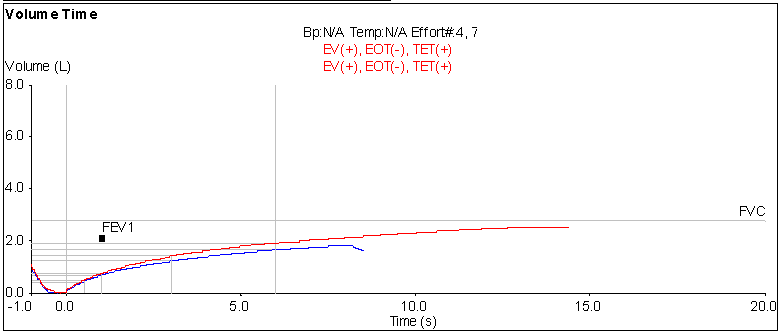
The patient whose gender raised this issue has relatively severe lung disease and is probably not the best example for this, but its what’s in front of me right now.
| Female: |
Observed: |
%Predicted: |
Predicted: |
| FVC: |
1.37 |
42% |
3.25 |
| FEV1: |
0.92 |
36% |
2.54 |
| FEV1/FVC: |
67 |
84% |
80 |
|
|
|
|
| TLC: |
2.69 |
54% |
4.93 |
| RV: |
1.34 |
79% |
1.69 |
|
|
|
|
| DLCO: |
14.90 |
78% |
19.12 |
Continue reading →