A web link to this photo was generously provided by Alan Wyatt, BS, RRT-NPS.

Bernstein Spirometer 1969, from Drew CDM, Hughes DTD. Characteristics of the Vitalograph Spirometer,Thorax, 1969; 24: 703

Vitalograph Spirometer, 1969. From Drew CDM, Hughes DTD. Characteristics of the Vitalograph Spirometer,Thorax, 1969; 24: 703
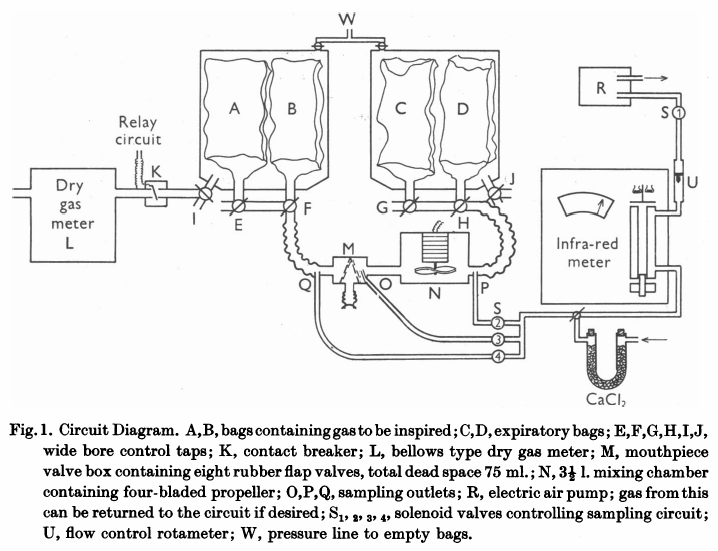
From Bates DV, Boucot NG, Dormer AE. The pulmonary diffusing capacity in normal subjects. J Physiol 1955; 129: 237-252
“The mouthpiece (M) contains eight rubber flap valves, four on the inspiratory and four on the expiratory side. Its dead space, including the flexible attachment to the mouth, was 75 ml Beyond the expiratory valves, a mixing chamber (n) of 3-1/2 L capacity contains an induction motor drive four propeller blades. Switching of the sampling circuits is done by solenoid valves (S) in appropriate places in the circuit. Gas analysis was by infrared analyzer of the type described previously (Lawther & Bates, 1953). This was calibrated on two scales, 0-0.15% CO and 0-0.05% CO. The accuracy of this type of instrument is about 3% of scale reading. Gas was drawn through this by a small electric air pump (R), at a rate of 2 l/miin, measured by a rotameter (U). Sampling could be instantly stopped by closing solenoid S1, which was energized through a relay circuit connected to a contact breaker (K), so that, if required, gas was sampled from just beyond the expiratory valve only during inspiration. All exercise was performed on a motor-driven treadmill, the gradient of which was adjustable. When ‘flat’ it actually sloped at a grade of 1:50. At ‘1/2’ slope the grade was 1:10.7. All tubing in the direct breathing parts of the circuit was 5.1 cm in internal diameter and the three-way taps (E to J) had a minimum diameter internally of 3.5 cm. During studies on maximum exercise with ventilation rates up to 90 l/min, the gas meter was disconnected from the circuit and the minute volume measured from the volume of gas collected in the expiratory bag. The pressure swing at the mouthpiece in these circumstances was from -2.6 to +3.4 cm H2O.
“Two initial ‘calibrating’ experiments were performed. The first set of observations consisted of attaching a hand-operated pump of constant stroke volume to the mouthpiece and checking that the gas displaced from the bag containing inspired gas (A or B) into bag C, corresponded with the volume recorded by the gas meter. This volume was measured by wet drum flowmeter, the gas being expressed by positive pressure on the tank at W. The second set of observations compared the mixed expired CO concentration as measured by continuously sampling gas from P, with that of the simultaneously collected expired gas in bag D. For seventeen such experiments, the bag gas averaged 0.002%CO less than the continuous readings. This is just outside the reading error of the meter, and was presumed to results from incomplete bag emptying before the experiment began. Since, however, this error is less than 2% of the average mixed expired CO, it has been disregarded in subsequent experiments. The experiments were performed at different rates of breathing, and at all times the CO meter gas a steady reading on continuous sampling from P; it can be assumed that the box N ensures complete homogeneity of mixed expired gas during these experiments.
“Before each experiment, the infrared meter was set to zero on dry air and its sensitivity checked against a standard cylinder of 0.04% CO. As described later, a zero correction was made during each experiment for ‘interference’ due to water vapour and expired CO2. The deflexion produced by these factors varies with state of ‘decay’ of the detector of the instrument and must be constantly checked.
“The procedure followed in using this circuit varied slightly in different experiments. In those of which the results are shown in Table 2, the subject sat quietly at rest in a chair for 5 minutes. He was then connected to the circuit, the taps being adjusted so that he breathed room air. With the fan N in operation and solenoids LS1 and S2 open o that the meter is sampling continuously from P, a zero reading (Z) is taken on the CO meter. Solenoid S2 is then closed and solenoid S4 is opened, and the subject is switched into 0.14% CO in air contained in bags A and B. The inspired gas concentration (F1) is read on the meter. S4 is closed and S2 again opened, and the mixed expired CO concentration is noted. This usually becomes steady in 1 min or less, but may vary either due to the fact that an equilibrium state had not been reached or due to variations in ventilation. The next stage in the experiment is delayed until a steady mixed expired concentration is being recorded. The fan N is now turned off, and solenoid S2 is closed and S3 is opened. Solenoid S1 is connected to the relay circuit actuated by K. Tap I is now turned so that the gas meter is connected to the inspired gas tank containing bag A and B. At the same moment tap G is turned o all expired gas is collected in bag C; this gas is analyzed for CO2 and O2 so that the appropriate R.Q. correction can be applied. For the next minute (timed by stopwatch) the gas meter will read the minute volume, and the meter will sample from O during each inspiration only.”
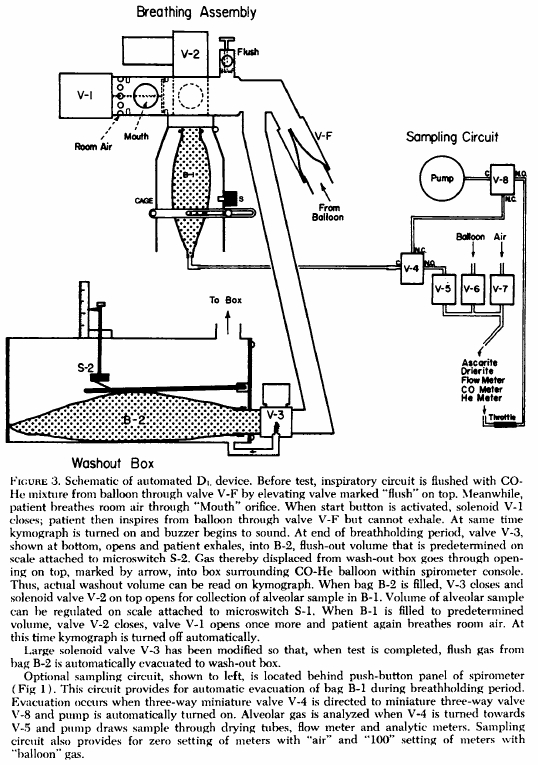
From Gaensler EA, Smith AA. Attachment for automated single breath diffusing capacity measurement. Chest 1973; 63: 136-145.
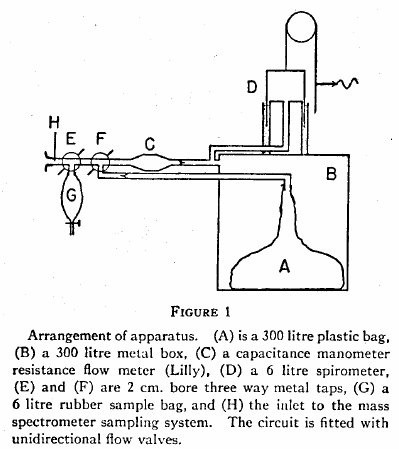
From Forster RE, Fowler WDS, Bates DV, Van Lingen B. The absorption of carbon monoxide by the lungs during breathholding. J Clin Invest 1954; 33: 1135-1145
“Inspiration was made from a neoprene bag (A) contained in a sealed metal chamber (B). Expiration was made through the flowmeter (C) into chamber (B). A 6-liter spirometer (D) recorded changes of total volume in the system. Two 2 cm. diameter three-way taps (E and F) were provided. Tap E enabled the subject to breath out the first liter of an expiration into the circuit, and to collect the remainder in a rubber bag (G) close to the mouthpiece. Tap F permitted closure of the inspiratory bag to prevent loss of the inspired gas. The inlet (H) to the mass spectrometer sampling tube was at the mouthpiece.”
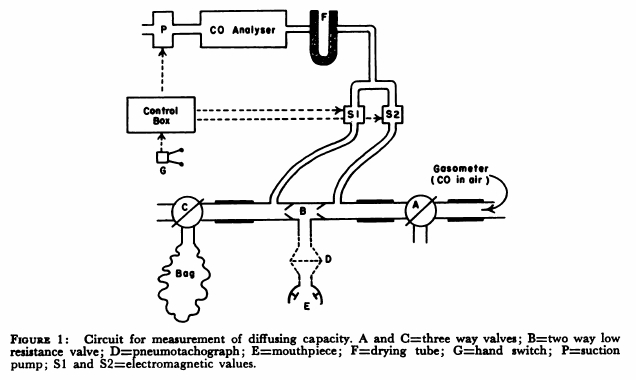
From: Woolf CR. An assessment of the Fractional Carbon Monoxide uptake and its relationship to pulmonary diffusing capacity. Chest 1964; 46: 181-189.
“The technique used to measure diffusing capacity is a modification of the end tidal steady-state method described by Bates. The arrangement of the apparatus is shown in Fig.1. The patient, comfortably seated, breathed through a two-way low resistance valve (B) with the three-way valves (A and C) open to the air. By means of a switch on the control box an electromagnetic valve (S1) was opened and a a small diaphragm suction pump was activated causing expired air to be drawn continuously through a Liston-Becker carbon monoxide analyzer. The reading on the meter of the analyzer gave the correction to be made for expired carbon dioxide. Valve A was turned so that the patient breathed from a recording 350 liter gasometer containing approximately 0.13 per cent carbon monoxide in air. The inspired carbon monoxide concentration was obtained by opening electromagnetic valve S2 and drawing the gas through the analyzer. When a steady reading had been obtained, valve S2 was closed. End tidal gas was obtained by opening valve S1 and activating pump P with a hand switch G during each inspiration. Repeated end-tidal samples were drawn through the gas analyzer until a steady reading for end tidal carbon monoxide concentration was obtained. Then valve C was turned and mixed expired gas collected in a bag for one minute and this completed the test. The mixed expired gas carbon monoxide concentration was obtained by separately passing the bag gas through the carbon monoxide analyzer. Minute volume, respiratory rate and tidal volume were obtained from the recording drum of the gasometer.”
Henderson M, Apthorp GH. Rapid method for estimation of carbon monoxide in blood. Brit Med J 1960: 2: 1853
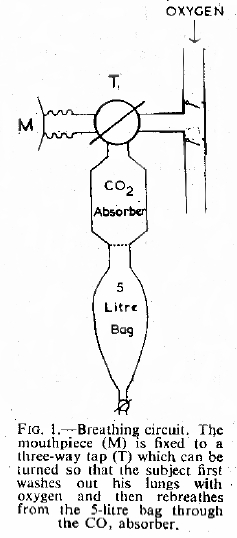
“Each subject washed the nitrogen from his lungs by breathing 100% oxygen from a simple open circuit (Fig. 1) for a period of three minutes. At the end of this time he was instructed to take a maximum inspiration and hold his breath. The three-way tap (T) was then turned and exhaled through a carbon dioxide absorber, previously washed out with oxygen into an empty and rebreathed from this bag for a further three minutes. The contents of the bag were then analysed for oxygen by the Haldane method and for carbon monoxide, using an infra-red meter.”
From: Marshall R. A rebreathing method for measureing carbon monoxide diffusing capacity. Amer Rev Resp Dis. 1977; 115: 587-588
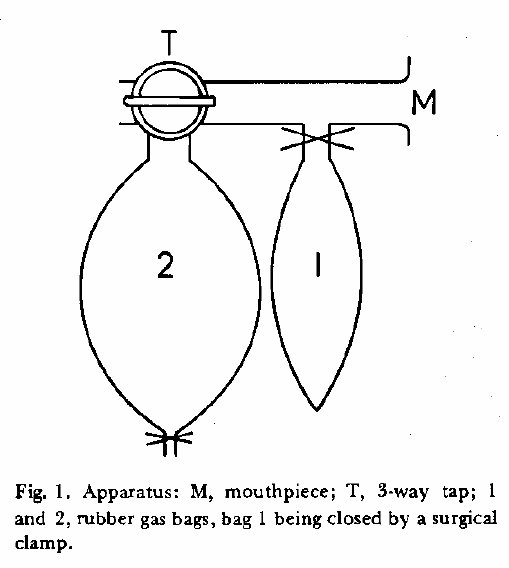
“The apparatus consists of a mouthpiece, A T-piece to which bag 1 is attached by a short length of 1.2 cm bore rubber tubing that can be closed by a surgical clamp, and a 3-way tap, to the side arm of which the reservoir bag (bag 2) is attached. Bag 2 should be able to contain about 3 liters and bag 1 500 ml when just distended. Measurement of the capacity of bag 1 is important because the intention is just to distend the bad when collecting sample 1 so that the volume of gas removed from the re-breathing circuit is known.
“Into bag 2 is measured accurately (by gas syringe) 2 liters of the helium-CO mixture used for the single-breath method and 100 ml of oxygen. This volume of oxygen is added to replace most of the oxygen that will be consumed during rebreathing. The subject with noseclip attached breathes quietly through the mouthpiece. At the end of a normal expiration the tap is turned to connect bag 2 and the subject is instructed to breath rapidly, almost emptying the bag at each breath. At about the tenth second, most conveniently at the start of an expiration, the clamp on bag 1 is released and gentle pressure is applied to bag 2 to stop it filling so that the expired gas goes into bag 1 and just fills it. The tube to this bag is then re-clamped. The subject is encouraged to breathe hard and exactly 20 sec (or similar time interval measured by a stop watch) the tap is turned to close off bag 2. The contents of bags 1 and 2 are analyzed for CO and helium.”
From Forster RE, Roughton JW, Cander L, Briscoes WA, Kreuzer F. Apparent pulmonary diffusing capacity for CO at varying alveolar O2 tensions. J Appl Physiol 1957; 11: 277-289
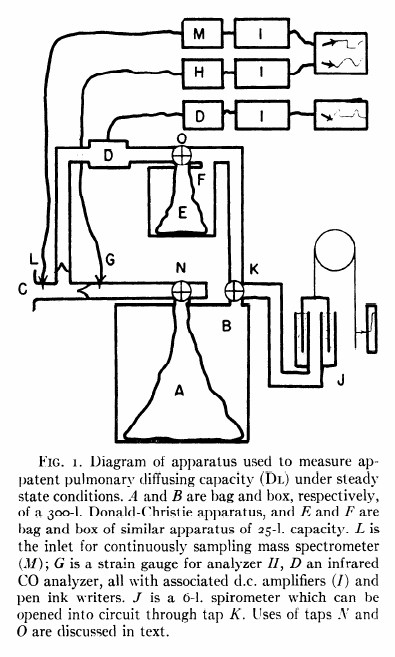
“The subject inspired the CO mixture from the bag (A) of a 300-liter Donald-Christie bag-in-box (A, B) through a mouthpiece (C) with unidirectional valves. The expired gas passed through an infrared CO analyzer (D) and next to a 30-liter bag-in-box (E, F), which was used to collect the expired gas. It then returned to the 300 liter box. Since the entire circuit is closed, small pressure changes occur during breathing and these are recorded by a sensitive strain-gauge (G), analyzer (H) and associated amplifier (I) and pen ink writer. As the volume of the entire circuit is more than 300 liters, the gas pressure changes during normal breathing are less than 1/400th atmosphere and are proportional to the respiratory volumes. Under the conditions employed the respiratory volume record was accurate to better than 25 ml. The advantage of this system or recording respiratory gas volumes was that it had negligible time delay (less than 0.01 sec). A spirometer (J) was included in the apparatus, but was normally cut off from the main circuit by tap K. This spirometer was used a) to record and compensate for any large changes in end-expiratory volume, as it is more comfortable for the subject if the average pressure in the circuit approximates atmosphereic, and b) for calibrating the pressure record in terms of colume changes. CO2 concentration of the gas at the mouthpiece was recorded bu a continuously sampling mass spectrometer (sampling rate of 6 ml/min), which was precise to 0.05% CO2 and had a 90% response to a stepwise change in gas concentration at the sampling inlet (L) of 0.06 sec. CO concentration in the expired gas was measured in a chamber of low gas flow resistance (D) which was sensitive to as little as 0.0005% CO, was precise to 2% of a given CO concentration and had a 90% response time of 0.4 seconds. In addition to it slower response time, 250 ml of gas were required to flush out the mouthpiece, sample chamber and the associated tubing. During inspiration, however, no gas passes through the analyzer and the instrument has therefore ample time to register the true CO concentration of the final portion of the previous expiration. This value is taken to be mean alveolar concentration. The infrared analyzer is also sensitive to H2O and CO2. Fortunately, the effects due to these gases were additive and were thus easily allowed for. The strain gauge manometer, mass sprectrometer and CO analyzer all recorded through amplifiers (I) on magnetic pen ink writers.”