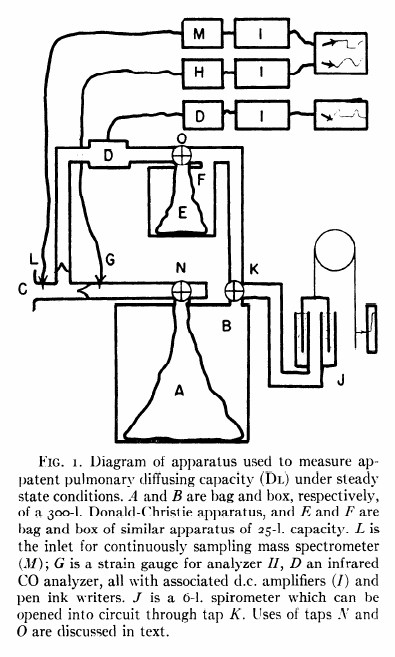
From Forster RE, Roughton JW, Cander L, Briscoes WA, Kreuzer F. Apparent pulmonary diffusing capacity for CO at varying alveolar O2 tensions. J Appl Physiol 1957; 11: 277-289
“The subject inspired the CO mixture from the bag (A) of a 300-liter Donald-Christie bag-in-box (A, B) through a mouthpiece (C) with unidirectional valves. The expired gas passed through an infrared CO analyzer (D) and next to a 30-liter bag-in-box (E, F), which was used to collect the expired gas. It then returned to the 300 liter box. Since the entire circuit is closed, small pressure changes occur during breathing and these are recorded by a sensitive strain-gauge (G), analyzer (H) and associated amplifier (I) and pen ink writer. As the volume of the entire circuit is more than 300 liters, the gas pressure changes during normal breathing are less than 1/400th atmosphere and are proportional to the respiratory volumes. Under the conditions employed the respiratory volume record was accurate to better than 25 ml. The advantage of this system or recording respiratory gas volumes was that it had negligible time delay (less than 0.01 sec). A spirometer (J) was included in the apparatus, but was normally cut off from the main circuit by tap K. This spirometer was used a) to record and compensate for any large changes in end-expiratory volume, as it is more comfortable for the subject if the average pressure in the circuit approximates atmosphereic, and b) for calibrating the pressure record in terms of colume changes. CO2 concentration of the gas at the mouthpiece was recorded bu a continuously sampling mass spectrometer (sampling rate of 6 ml/min), which was precise to 0.05% CO2 and had a 90% response to a stepwise change in gas concentration at the sampling inlet (L) of 0.06 sec. CO concentration in the expired gas was measured in a chamber of low gas flow resistance (D) which was sensitive to as little as 0.0005% CO, was precise to 2% of a given CO concentration and had a 90% response time of 0.4 seconds. In addition to it slower response time, 250 ml of gas were required to flush out the mouthpiece, sample chamber and the associated tubing. During inspiration, however, no gas passes through the analyzer and the instrument has therefore ample time to register the true CO concentration of the final portion of the previous expiration. This value is taken to be mean alveolar concentration. The infrared analyzer is also sensitive to H2O and CO2. Fortunately, the effects due to these gases were additive and were thus easily allowed for. The strain gauge manometer, mass sprectrometer and CO analyzer all recorded through amplifiers (I) on magnetic pen ink writers.”