There have been numerous criticisms of the single-breath DLCO technique, many of them quite valid. In particular, the standard equation for calculating DLCO makes no consideration for the inspiratory and expiratory phases of the maneuver when lung volume and the alveolar capillary surface area are changing. Some investigators have devised ways of correcting for inhalation and exhalation, however other investigators have sidestepped the issue entirely by showing that DLCO can instead be calculated from information acquired only during exhalation.
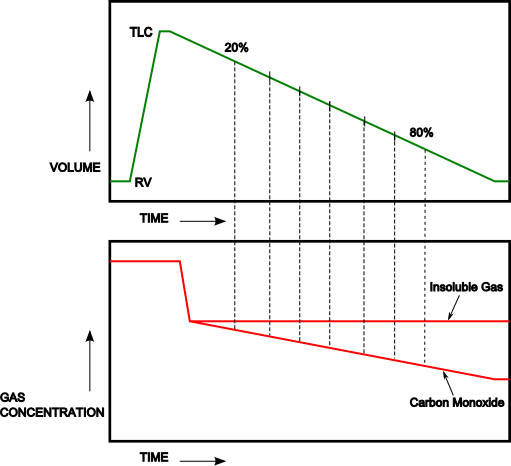
During exhalation, once the gas that exits the lung leaves the alveoli, diffusion ceases. Gas that exits the lung during the early part of exhalation will have had less time for CO to diffuse from the alveoli into the pulmonary capillaries and will have a higher concentration of carbon monoxide than will gas that exits later. Exhaled gas can therefore be considered to consist of a continuous set of alveolar gas samples, separated by time and the differences between these “samples” can be used to calculate DLCO.
Several techniques have been developed to calculate DLCOexh. What these techniques have in common is that they all use a relatively standard single-breath DLCO gas mixtures in conjunction with fast responding carbon monoxide and helium or methane gas analyzers. They also require the subject exhale slowly (approximately 0.5 L/sec) after inhaling the DLCO gas mixture to TLC. The primary differences between these techniques lies in the way DLCOexh is calculated.
DLCOexh – Point Sample Technique:
In the study of Newth et al [9] the carbon monoxide and helium gas concentrations were determined for the midpoint of each 10% decrement in lung volume from 20% to 80% of the subjects exhaled volume.