Paramagnetic oxygen analyzer. Mechanism was a glass dumbbell suspended by a glass thread between the poles of a magnet. The amount by which the dumbbell rotated depended on the amount of oxygen in the sample chamber. A light reflected off a mirror attached to the dumbbell and showed up as a light spot on the front panel display. The black bulb in the rear was used to pull a gas sample into the analyzer. Photo is courtesy of Jim Sullivan, Supervisor, Pulmonary Diagnostic Laboratories at Memorial Sloan-Kettering Cancer Center.
Category Archives: Gas Analyzer
Oxygen Analyzer, Critikon, 1970’s
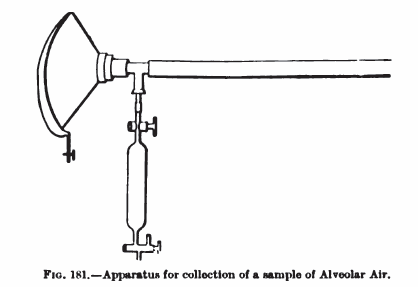
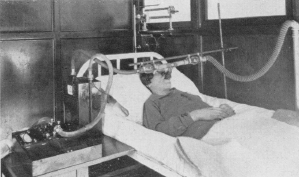
Alveolar air sampler, 1910
From: Practical Physiology, Edited by M.S. Pembrey, Published by Longmans, Green and Co., NY, 1910, page 183
“The composition of the alveolar air is determined, according to the method introduced by Haldane and Priestley, by an analysis of the last portion of the air expired in an ordinary expiration. The experiment may be performed in the following way. An anaesthetic mask is connected by a T-piece to a piece of tubing 80 cm long and 1.8 cm internal diameter; to the free end of the T-piece is connected (Fig. 181) a gas sampler with a capacity of 50 cubic centimeters. The subject of the experiment fits the mask to his face and makes an ordinary expiration; as soon as the expiration ceases, the tap of the gas-sampler, the air of which has previously been removed be a vacuum-pump or gas-pump, is opened and a sample of the last portion of the expired air is collected before the mask is removed from the face. The analysis of the air is performed in the manner already described. The percentage composition is about 5.5 carbon dioxide, 14.5 oxygen and 80 nitrogen.
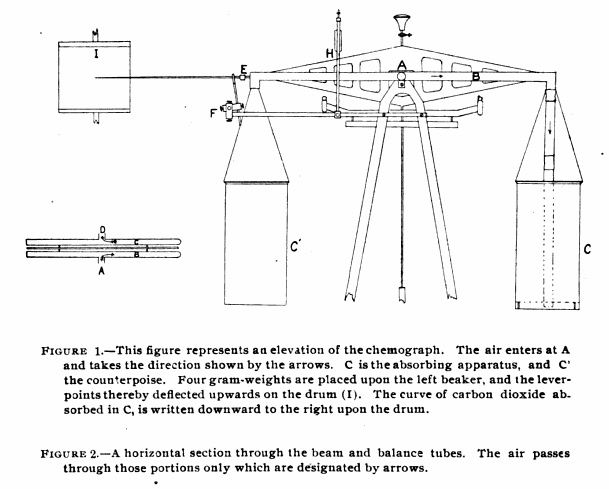
Balance Chemograph, 1905
From: A balance-chemograph and the excretion of carbon dioxide during rest and work, by George Oswin Higley, University of Michigan PhD Dissertation, 1905, page 6.
Before the infrared absorption CO2 analyzer was invented CO2 was analyzed by being absorbed chemically and the change in weight measured.
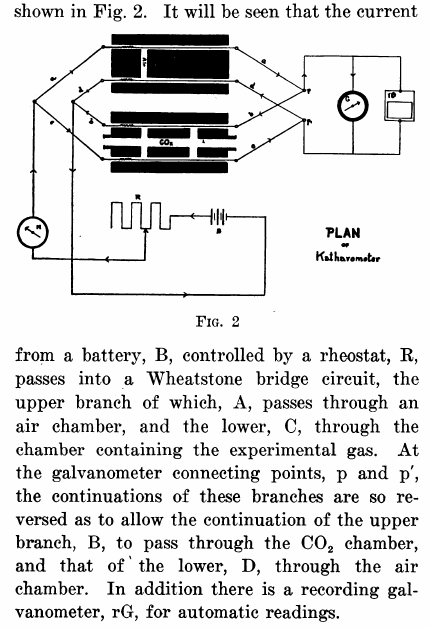
“The apparatus for absorbing carbon dioxide and recording on a blackened paper its rate of flow, is constructed as follows: (Fig. 1, Elevation). It consists of a Ruprecth lecture-room balance, capable of carrying a load of 6 kilograms in each pan and of turning to 5 milligrams. To the beam there was attached a copper tube one and one-hal centimeters in diameter as shown by figure 2. Dry air containing carbon dioxide, enters at A through a short piece of very thin rubber tubing made of a surgeon’s finger cot, passes through the portion designated by the arrows to the end of the beam and downward through two rubber connections like that just mentioned, and a glass tube D (Fig. 1) to the chamber for the absorption of carbon dioxide, upon the air of the balance. (C Fig 1.) From the absorption apparatus the air passes upward through similar connections to the balance-tube C, back on the opposite side of the balance-beam to the center, where it leaves the balance through another piece of rubber tubing, and then passes into guard tubes G’ and G”, which will be described later.
“Since there was a question of removing the carbon dioxide from air flowing at the rate of 30 liters per minute during work, the absorption apparatus is necessarily large. It consists of a beaker 20 centimeters deep, with cover of thin copper, provided with opening two centimeters in diameter, into which are fitted the inlet and outlet tubes. The air passes downward into the beaker through a thin glass tube 2 centimeters in diameter, to within about 2 centimeter of the bottom of the beaker, ending in a open space 3 centimeters deep and of a diameter equal to that of the beaker. (This open space was left because it was thought that the carbonic acid gas would thereby be more uniformly distributed throughout the whole cross-section of absorbent placed above.) The air now rises through the 5 kilograms of coarse, carefully screened soda-lime, and then through glass-wool covered with phosphorus pentoxide to hold back dust and the last trace of water formed in the reaction. This beaker when charged weighs about 5-1/2 kilograms. It is counterpoised by another beaker of the same exterior volume filled with spent soda-lime.”