I’ll start by saying that I am not associated with Hans Rudolph Incorporated in any way. I just think they make a bunch of good products, and more specifically in this case, a DLCO simulator.
For as long as I can remember I have always had intermittent problems with DLCO testing and I suspect that every PFT Lab has had them at some time. One of my current concerns has to do with how our test system’s software is calculating exhaled CO and CH4 concentrations from calibration data, but I’ve had other concerns at other times too. One way around to verify that your test system is actually performing the way it should is biological QC but a more precise way is to use a DLCO simulator.
Every PFT Lab should be performing biological QC (self-testing) regularly (and if you aren’t, why aren’t you?). This is the simplest way to check a test system but it does have limited accuracy. My personal experience for DLCO tests is that there was usually a range of about 1.5 ml/min/mmHg (roughly 5%) within the results from a single testing session and 2.5 ml/min/mmHg within session-averaged results over the course of a year. This is good enough to get a general sense of how well a test system is working but not precise enough to pinpoint specific problems. Used correctly a DLCO simulator can produce highly reproducible and accurate test results.
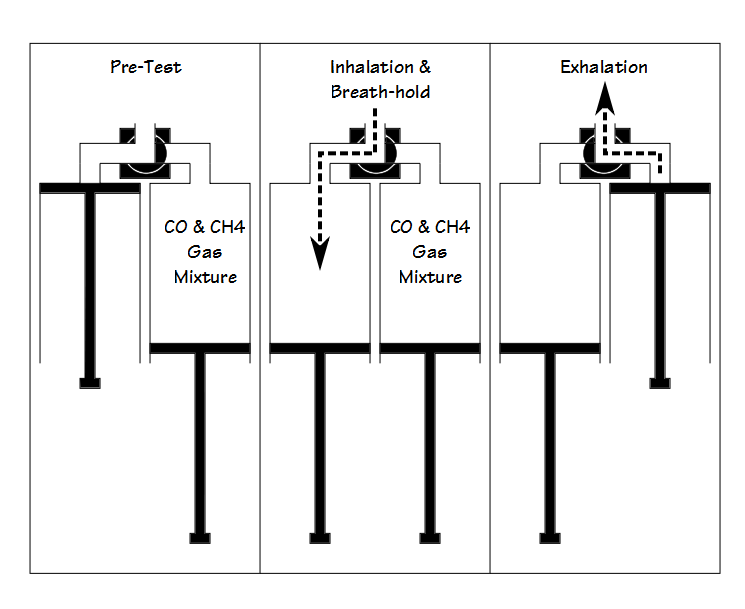
The Hans Rudolph 5560 DLCO Simulator was developed and patented by Robert Crapo and Robert Jenson (US Patent 6,415,642) and is conceptually quite simple. Two calibration syringes are connected through a Y-valve. A 5-liter syringe is used to simulate the patient inhalation portion of the DLCO maneuver and can be preset for inhalations from 2.0 to 5.0 liters. The other syringe, filled with a gas mixture with known CO and CH4 concentrations, is used to simulate the patient exhalation.
Using the simulator does require a bit of practice and manual dexterity. Three different gas mixtures, representing high, medium and low DLCO results are available from Hans Rudolph for the simulator. Assuming the inhalation syringe volume and the simulated exhaled gas mixture are the same, the biggest difference from test to test in calculated DLCO will likely be due to differences in “breath-holding” time. The DLCO test can be re-calculated manually with a standard 10 second breath-holding time, but strictly speaking reproducibly can be assessed just as well by looking at VA and the “exhaled” CO and CH4 concentrations.
When looked at over time the measured DLCO, VA and “exhaled” CO and CH4 concentrations measured with the simulator can be affected whenever the DLCO test gas is changed. A test system’s gas analyzer is calibrated with the assumption that the test gas mixture is 0.3% CO and that the insoluble component (CH4, He, Ne) is at its standard concentration. Test gas mixtures always vary from the standard concentration by a greater or lesser amount and this means is that the gas analyzer gain will differ from one tank of test gas mixture to another. Although these changes will not tend to affect patient test results (if you look carefully at the DLCO equation you will see it depends more on ratios than on absolute concentrations) any changes in analyzer gain will also affect the measured “exhaled” gas mixtures. Even so, the measured values from the DLCO simulator will likely remain far more stable and more reproducible over time than biological QC results.
QC results need to be recorded and trended. You can use a spreadsheet to do this or a program like Easylab QC (I haven’t used it so I can’t recommend it one way or the other). A normal range needs to be established and results reviewed regularly. When QC results start to trend out of normal limits this is often a sign of test system problems that may not appear when calibrating.
It may be tough to sell your hospital administration on the cost of a DLCO simulator. One approach may be to point out that the hospital’s chemistry and hematology labs perform QC all the time and you need to be able to as well. If your lab is involved with any research then QC is going to be mandatory and you will need to be able to provide trended QC results to show your lab is producing accurate results.
I am concerned that QC, whether biological or from a DLCO emulator, is not given the importance it deserves. It’s a critical part of running a quality PFT Lab and it should not be overlooked or ignored. Saying you don’t have time just means you need to build it into your schedule. Regular calibrations are a beginning step (and if you’re not doing regular calibrations then shame on you), but calibrations only test individual components. QC is a test of the entire system and the only way to assure that the results you report are “real” and accurate.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.