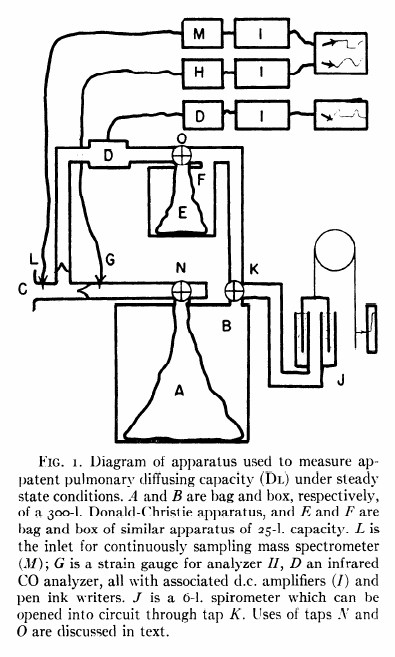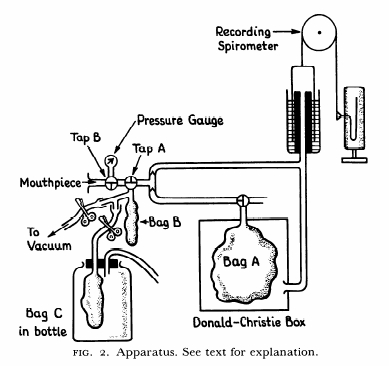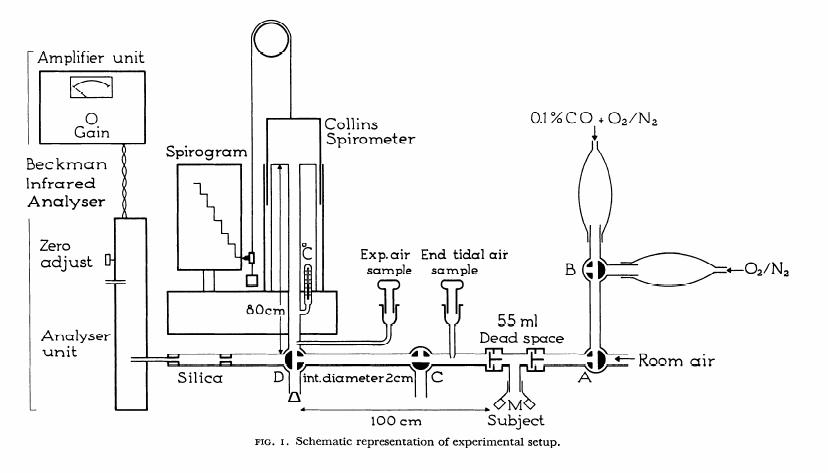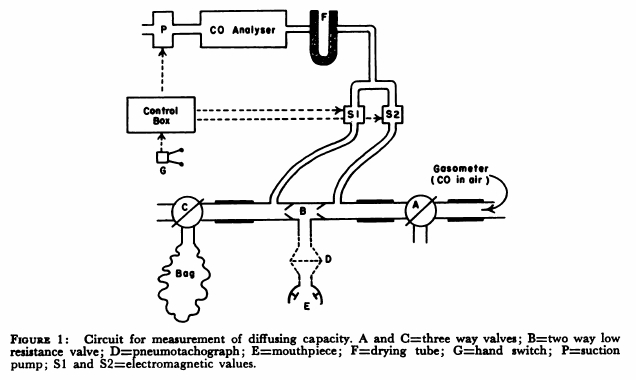
From: Woolf CR. An assessment of the Fractional Carbon Monoxide uptake and its relationship to pulmonary diffusing capacity. Chest 1964; 46: 181-189.
“The technique used to measure diffusing capacity is a modification of the end tidal steady-state method described by Bates. The arrangement of the apparatus is shown in Fig.1. The patient, comfortably seated, breathed through a two-way low resistance valve (B) with the three-way valves (A and C) open to the air. By means of a switch on the control box an electromagnetic valve (S1) was opened and a a small diaphragm suction pump was activated causing expired air to be drawn continuously through a Liston-Becker carbon monoxide analyzer. The reading on the meter of the analyzer gave the correction to be made for expired carbon dioxide. Valve A was turned so that the patient breathed from a recording 350 liter gasometer containing approximately 0.13 per cent carbon monoxide in air. The inspired carbon monoxide concentration was obtained by opening electromagnetic valve S2 and drawing the gas through the analyzer. When a steady reading had been obtained, valve S2 was closed. End tidal gas was obtained by opening valve S1 and activating pump P with a hand switch G during each inspiration. Repeated end-tidal samples were drawn through the gas analyzer until a steady reading for end tidal carbon monoxide concentration was obtained. Then valve C was turned and mixed expired gas collected in a bag for one minute and this completed the test. The mixed expired gas carbon monoxide concentration was obtained by separately passing the bag gas through the carbon monoxide analyzer. Minute volume, respiratory rate and tidal volume were obtained from the recording drum of the gasometer.”