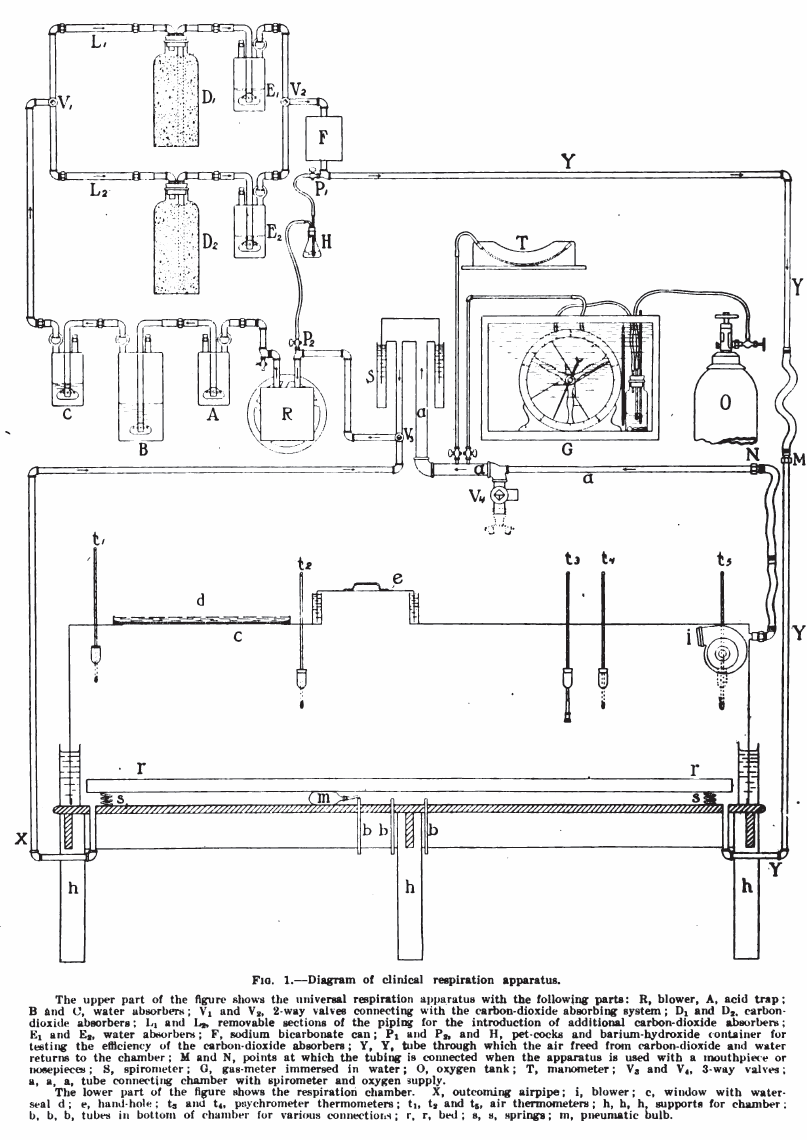
From: Studies in the respiratory exchange of infants, by Frances Gano Benedict and Fritz B. Talbot, The American Journal of Diseases of Children, Vol 8, July, 1914, page 25.
Category Archives: CO2 Production
Benedict Clinical Respiration Apparatus, 1916
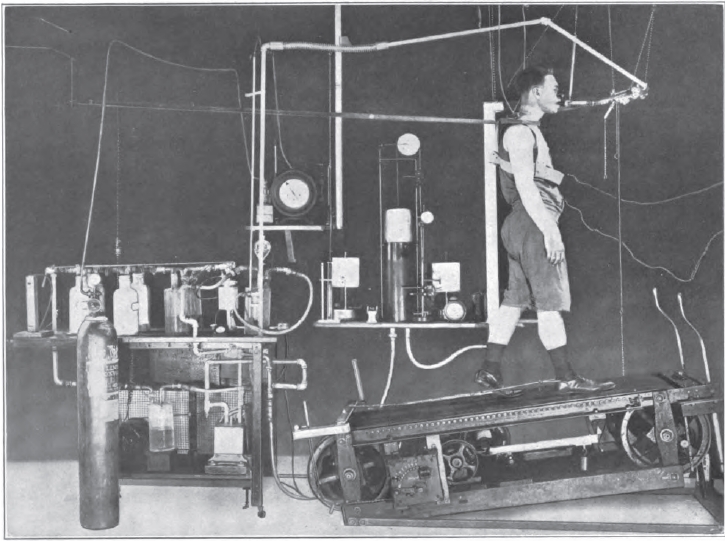
Exercise Testing, 1922
Gas collection system, 1889
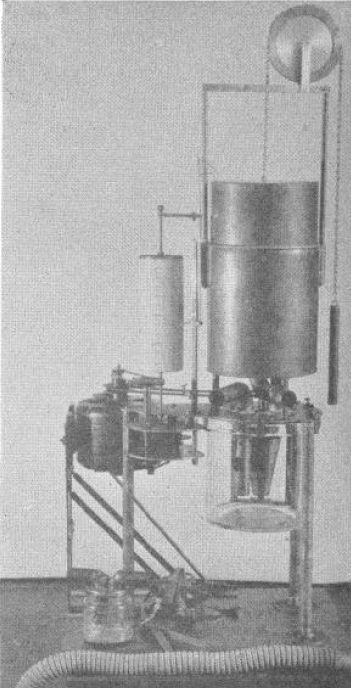
Spirometer, Portable Calorimeter, 1926
From: A portable calorimeter for the determination of both oxygen and carbon dioxide. JF McClendon, GJ Humphrey, MM Loucks. Journal of Biological Chemistry, 1926; 69: 513-517.
“The apparatus consists of a spirometer holding 6 liters and with a recording drum of such size that charts purchased from the Sanborn Corporation may be used to record the respirations. The bell is counterpoised by a chain and weight passing over a wheel on which the liters of oxygen in the bell may also be read if desired. Below the spirometer is a museum jar holding 6 liters in which is placed a standard solution of Ba(HO)2 containing BaCL2 to reduce hydrolysis and 0.1 gm. phenolphthalein, A synchronous alternating current electric motor drives a clock work revolving the recording drum and at the same time drives a centrifugal pump which sprays the Ba(HO)2 in the museum jar. The pump is made off a inverted, truncated hollow cone. The cone is open above and below and is provided with radial baffles or septa which allow passage of the fluid upwards but force the fluid to rotate with the cone. The rotation of the cone causes the fluid to be sucked up from the bottom and sprayed out at the top at a very rapid rate in order to absorb the CO2 out of the air.
“In the use of the apparatus a mask or mouthpiece is applied to the patient, having a 1 inch side outlet which allows free breathing to the outside until the moment of beginning the experiment. A 1-1/4 inch bore rubber tube leads to a brass tube of the same bore going down just below the level of the Ba(HO)2 solution in the museum jar, admitting expired air into the jar. The passage of the tube below the solution, acting as a valve, prevents reversal of the air current. From the museum jar a vertical tube rises up through the water in the spirometer. Another 1-1/4 inch brass tube comes down through the water of the spirometer and passes to the outside where it is connected with a valve and then to a 1-1/4 inch rubber tube to the mask over the patient’s face. This tube brings oxygen from the spirometer to the mask and the valve prevents reversal of the current. The apparatus is mounted on a stand of sufficient height to allow the museum jar to be raised up in place and clamped to the plate of the apparatus with six clamps, being made air-tight by a rubber gasket. In operation after the mask is applied (the bell having been filled with oxygen and the Ba(HO)2 solution having been introduced) the motor is started causing a base line to be written on the recording drum and the Ba(HO)2 rapidly sprayed in the museum jar. The patient is watched until the respirations become regular; then the side outlet to the mask is closed with a rubber stopper and the color of the Ba(HO)2 solution is carefully watched. As soon as the phenolphthalein is decolorized, the stopper to the side outlet of the mask is withdrawn, thus ending the experiment. The motor may be stopped and the mask removed at leisure.”