Recently my lab has had some turnover with a couple of older staff leaving and new staff coming on board. While reviewing reports I’ve found a number of instances where the incorrect FVC and FEV1 were reported. Taking these as “teachable moments” I’ve been annoying the staff with emails whenever I find something notably wrong. I had thought that our rules for selecting the best FVC and FEV1 were fairly straightforward but given the number of corrections I’ve made lately it seemed like it would be a good idea to revisit our policy on this subject.
The process I’ve used for selecting the best FVC and FEV1 has evolved over the years. Initially I was told to select the single spirometry effort that had the largest combined FVC and FEV1. Later on test quality became a factor (not that is wasn’t in the beginning but there aren’t a lot of quality indicators for a pen trace on kymograph paper). How to juggle the different quality rules wasn’t altogether clear however (they seemed to change a bit with whichever physician was reviewing PFTs at the time), and I was still supposed to somehow select just a single spirometry effort.
Most recently this was simplified by only having to select the largest FVC (regardless of test quality) from any spirometry effort and then the largest FEV1 as long as it came from a spirometry effort with good quality. This is pretty much in accord with the ATS/ERS spirometry standards but with one important difference, and that is that we use use Peak Expiratory Flow (PEF) as an indicator of test quality.
Strictly speaking the ATS/ERS standards state that
“The largest FVC and the largest FEV1 (BTPS) should be recorded after examining the data from all of the usable curves, even if they do not come from the same curve.”
There are, of course, a number of quality indicators for spirometry efforts that are used to indicate whether a curve is “usable”. These include things like back-extrapolation, expiratory time, terminal expiratory flow rate and repeatability but the one thing they do not include is PEF.
Despite not being within the ATS/ERS standards the reason that we use PEF in the selection process is found in the phrase “maximal forced effort” that is part of the ATS/ERS definition for FVC and FEV1. It has long been recognized (certainly since the early 1980’s and most likely earlier) that the FVC and FEV1 from a submaximal spirometry effort were often higher than the FVC and FEV1 from a maximal effort. So, is the largest FEV1 correct (as long as it meets the basic ATS/ERS criteria) or should it be the FEV1 from the effort with the highest PEF?
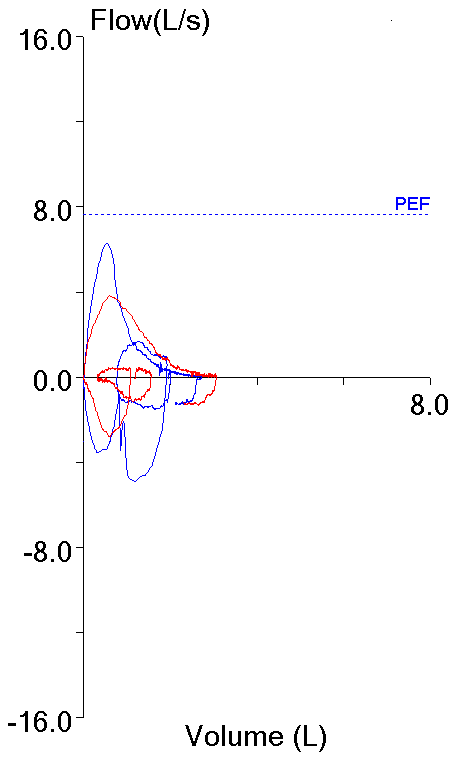
These two efforts from the same patient testing session highlight this dilemma. Both meet the ATS/ERS criteria for the start of the test which is what primarily applies to FEV1 (and PEF).
| Blue: | Red: | |
| FVC (L): | 2.72 | 3.06 |
| FEV1 (L): | 1.73 | 1.99 |
| PEF (L/sec): | 6.28 | 3.82 |
When transpulmonary pressure is measured (not exactly the same thing as alveolar pressure but close enough) during a forced exhalation, overall expiratory effort is related to the area under the curve of pressure versus volume. Some investigators have shown that the spirometry efforts with the highest PEF had the highest transpulmonary pressure-volume areas. Moreover, during a forced exhalation it takes about 250 milliseconds for the respiratory muscles to achieve maximal expiratory pressure. Peak flow therefore occurs while respiratory force is increasing and is largely dependent on patient effort. For these reasons PEF is a good index of expiratory effort.
Expiratory airflow is a function of driving (alveolar) pressure and the resistance to that flow, but only up to a point. Researchers have found that above a critical driving pressure expiratory flow rates either fail to increase or can actually decrease. This effect is usually called negative effort dependence (although a couple of researchers called it inverse effort dependence instead).
Part of the reason why an increased expiratory effort (and increased alveolar pressure) causes a decrease in expiratory flow has to do with thoracic gas compression. In order to generate a driving pressure the lung (thorax) has to be compressed and according to Boyle’s Law, this pressure is directly related to the amount of compression. A higher alveolar pressure therefore means more thoracic compression. More thoracic compression in turn means that the lung is actually at a lower volume than is apparent from the measured exhaled volume and therefore at a higher level of airway resistance and lower expiratory flow rates.
Negative effort dependence is usually more noticeable in subjects with airway obstruction and in general the worse the airway obstruction, the worse the negative effort dependence. The reason for this is mostly related to the equal pressure point. Since airways are elastic, they will be compressed when the pressure in the lung tissue surrounding them is higher than the pressure inside of them. This is the equal pressure point. Since airway elasticity depends to a large extent on the elasticity of the supporting lung tissue when that tissue loses elasticity due to disease processes, the amount of pressure necessary to collapse airways decreases as well. This is the reason why the maximum expiratory flow rate during quiet tidal breathing can be higher than the maximal expiratory flow rate at the same lung volume during a spirometry effort in individuals with COPD and also why a submaximal effort can produce a higher FEV1.
A number of studies have confirmed that PEF is related to effort and that FEV1 tends to be lower when taken from spirometry efforts with the highest PEF. Although at least one study showed these differences to be statistically significant, a couple other studies have shown the difference is marginal and not significant. Moreover is has been shown that FEV1 tends to correlate better with FVC than with PEF and that when FVC is factored in, there is no significant difference in the FEV1 from spirometry efforts with the largest PEF and those with a lower PEF. For these reasons selecting an FEV1 solely because that spirometry effort has the highest PEF doesn’t necessarily make sense.
Having said that, it remains reasonably clear that tests with a maximal or near maximal PEF are more likely to be maximal efforts. Because by definition FEV1 (and FVC) are supposed to be maximal, PEF should play some role in selecting which spirometry effort the FEV1 is taken from. If this is done however, when the largest FEV1 is in a spirometry effort with a PEF below the patient’s maximal PEF, how far below the maximal PEF is acceptable?
The ATS/ERS spirometry standards aren’t particularly helpful about this since PEF is discussed mostly as a stand-alone procedure and does not discuss the PEF obtained during spirometry except to define it in terms of the flow-volume loop. Moreover, acceptable ATS/ERS repeatability for PEF is an absolute value (0.67 L/sec or 40 LPM) without regard to patient gender, age or height. More pertinently a study on the reproducibility of peak flow showed that +/- 5% could be obtained in the majority of subjects however that study used peak flow meters and was not concerned about selecting FEV1.
When using PEF as part of the FEV1 selection or comparison process the closest to any kind of consensus is that a couple of researchers have used 90% of the maximal PEF as a cutoff. Although arbitrary, a 10% window seems like a reasonable starting point and for this reason my lab’s policy (written about 10 years ago) for selecting spirometry efforts states:
“Selection of a test should not be made solely on the basis of the best peak flow, but the selected test should be within 10% of the best peak flow out of all of the tests performed.”
The original problem I had noted while reviewing reports was that FEV1 is too often being selected simply from the effort with the highest PEF and that higher FEV1’s from efforts with similar PEF’s (well within 10% of the best PEF) were being ignored. This is mostly an education issue and I will continue to pester our staff until it goes away.
A variety of methods for choosing the “best” FVC and FEV1 have been proposed at one time or another. These include:
- The spirometry effort with the highest combined FVC + FEV1.
- Averaging the FVC and FEV1 from three acceptable efforts.
- Averaging the FVC and FEV1 from two efforts with the highest FVC + FEV1.
- The highest FVC and FEV1 regardless of which effort they came from.
The reason for some of these proposals has more to do with session to session repeatability than with whatever constitutes the “best” FEV1 and FVC. Even so, all of these methods has been studied and all show a reasonable level of intersession repeatability (although it should be no surprise that averaged results showed the highest reproducibility). There seems to be no reason therefore, not to attempt to define the “best” FEV1 as long as it is used consistently.
The ATS/ERS statement on spirometry does not include PEF as component in the FEV1 selection process and I think this is a mistake. Within limits PEF appears to be a reasonable way to assess how maximal or submaximal a spirometry effort may be. Choosing the largest FEV1 from a spirometry effort with a PEF within 10% of the highest observed PEF is admittedly somewhat arbitrary but it is an approach that will likely exclude submaximal spirometry efforts and prevent them from being reported.
References:
Aggarwal AN, Gupta D, Jindal SK. The relationship between FEV1 and Peak Expiratory Flow in patients with airway obstruction is poor. Chest 2006; 130(5): 1454-1461.
Brusasco V, Crapo R, Viegi G. Series ATS/ERS Task force: Standardisation of lung function testing. Standardisation of spirometry. Eur Respir J 2005; 26: 319-338.
Coates AL, Desmond KJ, Demizio D, Allen PD. Sources of variation in FEV1. Am J Respir Crit Care Med 1994; 149(2): 439-443.
Hegewald MJ, Lefor MJ, Jensen RL, Crapo RO, Kritchevsky SB, Haggerty CL, Bauer DC, Satterfield S, Harris T. Peak expiratory flow is not a quality indicator for spirometry. Peak expiratory flow variability and FEV1 are poorly correlated in an elderly population. Chest 2007; 131(5): 1494-1499.
Holcroft CA, Eisen EA, Sama SR, Wegman DH. Measurement characteristics of peak expiratory flow. Chest 2003; 124(2): 501-510.
Koyama H, Nishimura K, Ikeda A, Tsukino M, Izumi T. A comparison of different methods of spirometric measurement selection. Respiratory Medicine 1998; 92: 498-504.
Krowka MJ, Enright PL, Rodarte JR, Hyatt RE. Effect of effort on measurement of forced expiratory volume in one seconds. Am Rev Respir Dis 1987; 136: 829-833.
Nathan SP, Lebowitz MD, Knudsen RJ. Spirometric testing. Number of tests required and selection of data. Chest 1979; 76(4): 384-388
Park SS. Effect of effort versus volume on forced expiratory flow measurement. Am Rev Respir Dis 1988; 138(4): 1002-1005

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License

I saw one method that uses the sum of FVC +FEV1+1/2(PEF). Why only add half the PEF?
My guess would be that it’s to scale the effect PEF has on the selection process. Interestingly this approach does select the better quality effort (higher PEF) out of the two examples I put in the posting but I think it still tends to bias the selection towards the single effort with the largest PEF.
Thank you, Richard. Another excellent essay. We added ‘time to peak flow 0.12s unacceptable and out of contention for reporting FEV1s.
Best,
Kevin
Kevin –
I wouldn’t have thought to include time to peak flow, partly because I didn’t think there were any normal values for it. The only article that I’m aware of that looked at the time to peak flow is Miller MR, Pedersen OF, Quanjer PH. “The rise and dwell time for peak expiratory flow in patients with and without airflow limitation.” Am J Respir Crit Care Med 1998; 158(1): 23-27 and there they looked at the time to 90% of the peak flow not the peak flow itself. Do you have other references on this subject? FYI, I would also be concerned that the time to peak flow would be affected by back extrapolation, even when it was WNL.
Regards, Richard
Hi, Richard.
Another great treatment of an important topic.
Regarding Kat’s comments about using FVC plus FEV-1 divided by one-half of the peak flow, the actual equation used one-third of the peak flow, i.e., (FVC + FEV-1)/PEFR x .33). This was something SensorMedics had offered in the 1980s with their DOS software, and they called it ”QA Sum”. At that time, I thought it worked quite well because he was the first attempt anyone had made to include peak flow into the best effort decision.
I’ve probably responded to other posts with this before, but it’s worth repeating; spirometry is performed to assess the presence of airway obstruction, which is best revealed when the patient pushes down as hard and as sharply as they can on their airways. ”Hard” is the PEFR and “sharply” is the back-extrapolated volume; the hard they blow, the higher the PEFR and the more sharply they start the exhalation, the lower the Vext. I have long been a proponent of inclusion of the back-extrapolated volume and PEFR in the decision process of best effort. On patients with more than a mild degree of airway obstruction, I feel that the trial with the highest peak flow and the lowest back extrapolated volume is the best performed and most accurate measurement of airway function, even (or maybe especially) if the FVC and FEV-1 are lower in this trial. Accurate is the operative word; the largest isn’t the most accurate. I believe we’re reporting many overstated FVC and FEV-1 due to the current guidelines.