The issue of infection control has been a topic of a couple of discussions I’ve had lately. In particular, it was reported to me that a PFT lab had come under fire from a Joint Commission inspector who did not believe that filter mouthpieces were adequate and that “patient valves and circuits need to be sterilized between each patient”.
Unfortunately with all the other things we have to worry about it’s all too easy to become blasé about infection control. This despite the fact that every hospital I’ve visited in the last dozen or so years has posted numerous signs about hand washing and the safe disposal of contaminated supplies. But maybe it’s because we’re inundated with reminders that we’ve developed a blind spot about it.
The 2005 ATS/ERS statement on general considerations has two pages devoted to infection control (pages 155-157). The ATS procedure manual also has four pages devoted to infection control (pages 34-38), although much of this is devoted to a discussion of tuberculosis, cystic fibrosis and sterilization procedures. Of necessity, the ATS/ERS statement and ATS procedure manual discuss infection control in generalities and any given lab will need to have a policy tailored for their specific circumstances. Even so, either or both of these (as well as Kendrick et al’s 2003 review) should be the basis for your lab’s policy on infection control (and you do have one, don’t you?).
So what are the issues?
Diseases can be transmitted by direct contact (saliva) or indirect contact (airborne particles). PFT Labs need to prevent cross-transmission of diseases by the use of barrier devices (gloves, filter mouthpieces) and proper cleaning procedures.
So yeah, it’s as simple as that, but as usual the devil is in the details and in particular there are trade-offs between expense, time and efficacy.
Starting at the beginning, so to speak, of any pulmonary function test is the mouthpiece. Given that it is in direct contact with the patient’s saliva this item has the highest likelihood of transmitting diseases. A number of articles have suggested that when spirometry is performed as an expiratory-only maneuver, a cardboard mouthpiece is adequate.
Note: I am not a fan of expiratory-only maneuvers partly because coordinating both the maximal inspiration and inserting the mouthpiece at the beginning of the maneuver can be difficult for many patients (although there are some mouthpieces containing one-way valves that get around this); partly because despite instructions to do otherwise patients will still inhale from the spirometer; but most importantly I think that the pre-maneuver tidal breathing and a maximal inspiration after the maximal exhalation provide clinically important information.
A more effective approach would seem to be to use disposable filter mouthpieces but interestingly neither the ATS/ERS statement or ATS procedure manual mandates their use. There are appears to be at least two reasons for this. First, at least a couple studies have shown that filter mouthpieces can cause reductions in FEV1 and PEF. These reductions however, tend to be small and the general consensus has been that when low resistance mouthpiece filters are used these reductions are not clinically significant.
Second, the actual efficacy of mouthpiece filters remains unclear. Filter efficiency is usually measured at relatively low flow rates (usually 0.5 to 1.0 L/sec) and it’s unknown how well this translates to the flow rates routinely encountered during spirometry. On the plus side, at least one study using a mechanical model has shown that the possibility of bacterial cross-infection was zero when a filter mouthpiece was used. Another study however, that cultured swabs from actual mouthpieces showed that 2 out 155 mouthpieces had bacterial contamination on the distal (machine) side. The same study though, showed that 33 out of 155 mouthpieces had bacterial contamination on the proximal (patient) side which at least indicates that filter mouthpieces certainly reduces the potential contamination of the equipment.
Another study however, showed that a filter mouthpiece reduced downstream contamination but did not eliminate it. The authors did note however, that the mouthpiece filter chosen for the study had a lower efficiency rating than others in the marketplace. Interestingly though, the same study showed that a pneumotachograph also provided an additional filtering effect and when the two were in-line with each other that the downstream equipment was contaminated with measurable bacteria in only 1 out of 40 trials.
So a mouthpiece filter probably reduces the amount of equipment contamination but as both the ATS/ERS statement and ATS procedure manual state: “use of in-line filters does not eliminate the need for regular cleaning and decontamination of lung function equipment”. Unfortunately though, they do not indicate when and how often this should be done. Presumably this should be according to the manufacturer’s specifications but as an example, the manual for my lab’s equipment states:
“Under normal use, it is acceptable to clean the pneumotach at periodic intervals according to guidelines set by the hospital or laboratory’s infection control committee.”
Which pretty much leaves it up to us. I will note that more than one author has stated when mouthpiece filters are used tubing should be cleaned whenever there is a noticeable amount of moisture inside (and this presumably includes the manifold and valves) and this seems to be at least minimally adequate. A more stringent approach however, would be to do this daily but regardless some kind of regular cleaning schedule should be determined and adhered to.
But is it possible to sterilize mouthpieces and tubing, and to clean the test equipment between each patient visit and thereby bypass the need for disposable mouthpieces? Theoretically yes, and this has been a routine recommendation in the past. As an example, from Clausen’s 1982 textbook:
“All mouthpieces, tubing, valves and connectors from the patient to the measuring device should be disassembled, cleaned with detergent and water, thoroughly rinsed, and dried following each use.”
But this recommendation comes from a time when mechanical volume-displacement spirometers (both water seal and bellows) were the most commonly used type of equipment and it’s unclear how well this approach would work with the current crop of (more delicate?) flow-based test systems.
A cost analysis comparing the use of disposable filter mouthpieces versus cleaning after every patient showed that the cost for the time of the staff member cleaning the equipment and routine testing supplies was 5 times higher than the cost of disposable supplies (mouthpiece and nose-clip). This analysis did not take into consideration the cost of the extra inventory of non-disposable supplies that would need to be kept on hand in order to keep up with patient testing, nor did it explore the costs of automated cleaning equipment.
Note: There are a variety of cleaning and sterilizing systems intended for departmental-level use, and these range from systems using water and detergent, gas sterilization (ethylene oxide), steam sterilization and cold liquid sterilization (glutaraldehyde). All of these require a certain level of knowledge and experience to use and there are safety issues with some of them. In addition, there are significant up-front costs in acquiring them and for the supplies needed to operate them. Any lab that routinely requires sterilization of parts and testing supples should have this done in their hospital’s central supply department if for no other reason than because of their economies of scale.
In addition, whether or not this approach actually protects the patient any better than the use of a disposable mouthpiece filter has never been demonstrated. On the one hand a study showed that any aerosols from exhaled air were deposited on internal tubing within 5 minutes and that once deposited did not tend to become re-suspended. In addition, it was shown that five full flushes of a volume spirometer were sufficient to clear test systems of any suspended aerosols. On the other hand, studies from several decades ago showed that parts of the testing systems that were not amenable to routine cleaning (i.e. water seal spirometers) became contaminated relatively rapidly. It was never shown that this internal contamination had the potential to actually reach the patient, but in one reported case an individual cleaning a test system appeared to has acquired tuberculosis as a result of contact from a contaminated spirometer.
One completely novel and stringent approach to preventing cross-infection was proposed a while ago. Specifically, a bag-in-a-box system was developed using a disposable plastic bag and the authors were able to show that there was no significant effect on FVC and FEV1. Although an interesting idea, it never took hold.
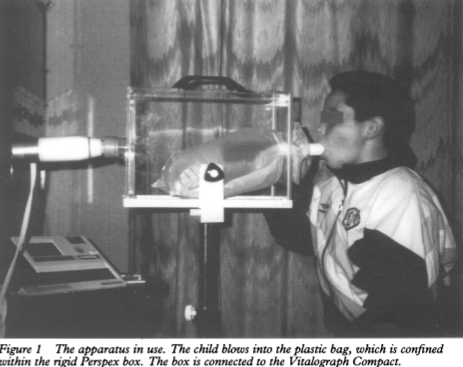
from Merchant J, Bush A. Prevention of cross-infection during outpatient spirometry. Arch Dis Child 1995; 72: page 156.
My personal recommendation is that disposable filter mouthpieces should be used with all patients and then disposed afterwards. In addition, when a flanged mouthpiece is needed, it too should be disposed of after use. This is within the ATS/ERS statement and ATS procedure manual guidelines and should significantly decrease (but not eliminate) the frequency that tubing, valves and manifolds will need to be cleaned. In addition, a filter mouthpiece provides the patient with some protection from any test system contamination.
So far, this has all been pretty much about mouthpieces. There are numerous other topics that apply to infection control in the PFT lab but many of these must be determined by individual labs based on their equipment, staffing and budget. The most important of these includes:
Handwashing: Staff must always wash their hands both before and after each patient session. The use of gloves during testing should also be considered.
Testing environment: Anything a patient is liable to touch should be cleaned with a germicidal wipe before the beginning of a testing session. This includes the patient chair and any part of the test system they will hold on to.
Test systems: Tubing, valves and manifolds should be cleaned regularly. Cleaning frequency will be determined by manufacturer instructions and visible indications, but should always be done on a regular basis.
Test supplies: All supplies needed for testing should be disposable whenever possible.
Patients: Each lab needs a policy addressing the testing of patients with known or suspected tuberculosis (and other communicable diseases), and for immunocompromised patients.
The actual level of risk of cross-infection from pulmonary function testing remains unclear and consists primarily of circumstantial, indirect and anecdotal evidence. The ATS/ERS statement indicates that the the risk of any cross-infection is low for any individual with a “competent immune system”. The ATS/ERS statement also states that there is “no direct evidence that routine pulmonary function testing poses an increased risk to immunocompromised patients.”
The problem is that it isn’t really possible to knowledgeably assign any level of risk to pulmonary function testing and these statements are guesses. Hospital-acquired infections are relatively common however. The CDC estimates that in 2011 (a year for which some of the best statistics are available) 721,800 individuals acquired an infection during a hospital stay (157,500 with pneumonia), and that 75,000 individuals with hospital-acquired infections died. Cross-infection from pulmonary function testing has to be responsible for at least some fraction of these.
Whether or not it was a single Joint Commission inspector being overbearing on a subject they weren’t knowledgeable about, PFT lab infection control is an issue that could easily be adopted by Joint Commission inspectors and become part of routine inspections. Although this is a reason to develop an infection control policy more pertinently it’s our responsibility to keep pulmonary function testing safe for all of our patients. For this reason alone we all need an infection control policy, and just as importantly, we need to follow it.
References:
Bracci M, Strafella E, Croce N, Staffolani S, Carducci A, Verani M, Valentino M, Santarelli L. Risk of bacterial cross infection with inspiration through flow-based spirometers. Am J Infect Control 2011; 39: 50-55.
Brusasco V, Crapo R, Viegi G. ATS/ERS Task force: standardisation of lung function testing. General consideration for lung function testing. Eur Respir J 2005; 26: 153-161.
Burgos F, Torres A, Gonzalez J, Puig de la Bellacasa J, Rodriguez-Roisin R, Roca J. Bacterial colonization as a potential source of nosocomial infections in two types of spirometers. Eur Respir J 1996; 9: 2612-2617.
Clausen JL. Pulmonary Function Testing Guidelines and controversies. Equipment, methods and normal values. Chapter 2, Gold PM, Schwesinger DW. Pulmonary laboratory infection control and safety. Published by Grune & Stratton, 1982.
Hancock KL, Schermer TR, Holton C, Crockett AJ. Microbiological contamination of spirometers. Australian Family Physician 2001; 41: 63-66.
Hiebert, T, Miles J, Okeson GC. Contaminated aerosol recovery from pulmonary function testing equipment. Am J Resp Crit Care Med 1999; 159(3): 610-612.
Johns DP, Ingram C, Booth H, Williams TJ, Walters EH. Effect of a microaerosol barrier filter on the measurement of lung function. Chest 1995; 107: 1045-1048.
Jones AM, Govan JRW, Doherty CJ, Dodd ME, Isalska BJ, Stanbridge TN, Webb AK. Identification of airborne dissemination of epidemic multiresistant stratins of Pseudomonas auruginosa at a CF centre during a cross infection outbreak. Thorax 2003; 58: 525-527.
Kamps AWA, Vermeer K, Roorda RJ, Brand PLP. Effect of bacterial filters on spirometry measurements. Arch Dis Child 2001; 85: 346-347.
Kendrick AH, Johns DP, Leeming JP. Infection control of lung function equipment: a practical approach. Resp Med 2003; 97: 1163-1179.
Merchant J, Bush A. Prevention of cross-infection during outpatient spirometry. Arch Dis Child 1995; 72: 156-158.
Normand H, Normand F, Le Coutour X, Metges M-A, Mouadil A. Clinical evaluation of a screen pneumotachograph as an in-line filter. Eur Respir J 2007; 30: 358-363.
Rasam SA, Apte KK, Salvi SS. Infection control in the pulmonary function test laboratory. Lung India 2015; 32: 359-366.
Side EA, Harrington G, Walters EH, Johns DP. A cost-analysis of two approaches to infection control in a lung function laboratory. Aust NZ J Med 1999; 29: 9-14.
Unstead M, Stearn MD, Cramer D, Chadwick MV, Wilson R. An audit of the efficacy of single use bacterial/viral filters for the prevention of equipment contamination during lung function assessment. Resp Med 2006; 100: 946-950.
Wanger, J. ATS Pulmonary Function Laboratory management and procedure manual, Third edition. Published 2016 by the American Thoracic Society.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
