Patients that have problems with oxygenation at sea level are going to have even more problems at higher elevations where the barometric pressure and oxygen partial pressure are lower. During commercial aircraft travel the cabin pressure is required by U.S. Federal regulations to be pressurized to at least 565 mm Hg which is the equivalent of 8000 feet altitude. It has been reported that most airliners are pressurized to an equivalent altitude of between 5000 and 8000 feet but this will depend on both the airplane and the airline in question.
There is a general relationship between a patient’s PaO2 at sea level and their PaO2 at altitude and a variety of studies have developed equations to predict an individual’s PaO2 at altitude using ABG, spirometry, DLCO and exercise SpO2 results. These prediction equations however, have been shown to have poor accuracy when compared to a Hypoxia Altitude Simulation Test (HAST).
The concept behind the HAST is relatively simple and that is to have the patient in question breathe a gas mixture containing a concentration of 15.1% oxygen (which at sea level is the equivalent to an altitude of 8000 feet) while monitoring the patient’s arterial oxygen partial pressure or saturation. The HAST has been shown to give the same results as a hypobaric chamber. Although HAST has been performed by a number of different research groups the technique has not been standardized which may to some extent account for some of the differences in reported results.
There is general agreement about which patients will need a HAST in order to ensure safe airplane travel and these are patients with a resting SpO2 between 92% and 95%, particularly ones with a comorbidity such as cardiac or pulmonary disease. Patients with a resting SpO2 above 95% will most likely not require supplemental oxygen and do not require a HAST. Patients with a resting SpO2 below 92% will likely require supplemental oxygen during airplane travel and will not require a HAST although a HAST can be used to titrate their supplemental oxygen flow rate.
There is also general agreement about the length of the HAST (20 minutes) and which HAST results that indicate the need for supplemental oxygen:
- PaO2 below 55 mm Hg
- SpO2 below 85%
The lack of standardization is in the nuts and bolts of how to perform the HAST. Delivery of the 15.1% oxygen gas mixture is most often by use of a tight-fitting mask but a mouthpiece with nose clips, filling a body box with the gas mixture or loose-fitting masks of different types have also been proposed as delivery techniques. Several years ago when my PFT lab decided to start performing this test we evaluated these different approaches and used ease of testing and low expense as primary factors in deciding how to perform the test. Part of the low-cost factor includes making as many of the components disposable after use because sterilization is relatively high cost for us.
Although a breathing circuit with a mouthpiece and nose clip is probably the simplest approach, one key component of the HAST is the need to be able to titrate supplemental O2 if it turns out to be needed. Since supplemental O2 is most often given by nasal cannula it’s not possible to titrate O2 with a mouthpiece and this factor alone made this approach unfeasible.
It might be possible to use a body box but the plethysmographs we have do not particularly lend themselves to this. At the very least a hole would have had to have been drilled in the wall of the box so that a nipple could installed as an inlet for the HAST gas mixture. Most plethysmographs come with either a solenoid valve or low-pass filter to keep the interior of the box at atmospheric pressure so an additional opening for outflow would not necessarily be a problem and a small oxygen tank and regulator could be placed in the box to deliver and titrate supplement O2.
The biggest drawback of this technique is that the volume of the box would require a large amount of the HAST gas mixture to flush and fill the box properly. Most plethysmographs have a volume on the order of 600 liters but it would probably take at least two to three times that volume in order to flush any residual room air. This may not be a problem for those labs that are able to mix the HAST gas mixture with a blender from oxygen and nitrogen (at least in terms of expense) but a blender would have been an extra expense for my lab. Significant additional test time to fill and flush the body box would also be needed while the patient is inside (even with a gas flow rate of 100 LPM it would take somewhere between 10 and 18 minutes to do this and I have to wonder about the noise level inside the box during this time). Finally the oxygen concentration inside the box must be monitored reasonably accurately as well (another expense for my lab) and if the gas concentration is over or under the 15.1% O2 goal even more time would be needed to correct it. All these factors ruled out the use of a body box for us.
This left us with the mask approach and to use a mask the first question would be what kind of mask? At least one group of investigators used a 35% venturi mask driven with nitrogen instead of by oxygen. My prior experience with venturi masks showed me that the gas concentration is dependent to some extent on driving pressure and that individual masks can have minor differences in the concentrations they provide. Since HAST results are sensitive to oxygen concentration using a venturi mask would have required a precision oxygen analyzer to verify that the correct oxygen concentration was being generated and for my lab this would have been an additional expense.
It’s possible that a simple non-re-breathing oxygen mask could be used and it would be easy for the patient to wear a nasal cannula underneath it but since it is loose fitting it would need a high flow rate of the HAST gas mixture (+30 LPM?) to make sure the patient got the right oxygen concentration. This in itself is not necessarily a problem but the high flow rate of gas into the mask would likely disturb the supplemental O2 when it was delivered by the nasal cannula by flushing it away before it was inhaled. This meant we couldn’t rely on the accuracy of the supplemental O2 flow rate needed to maintain a normal SpO2 and this factor ruled out this approach for us.
A number of relatively inexpensive tight-fitting masks in a range of sizes with a wide, low-pressure seals that would permit a nasal cannula to be placed underneath are available so the next question is how the HAST gas mixture should be delivered. The most economical approach in terms of the amount of test gas used would be to have a reservoir bag and a two-way breathing valve that the mask would be attached to. The problem with this that when a patient breathes in, they cause a slight amount of negative pressure inside the mask and any leaks around the edge of the mask (facial hair, sunken cheeks, fit around the bridge of the nose) would cause room air to be drawn inside, raising the oxygen concentration. Even a well-designed two-way valve would add extra resistance which would increase this negative pressure and therefore increase the likelihood of this happening. In addition, masks tend to impose an extra dead space load on the patient and tend to retain exhaled CO2. All of these factors ruled out a reservoir bag and two-way valve and led us to a mask with a blow-by system (or to be more accurate in this case, a blow-at system).
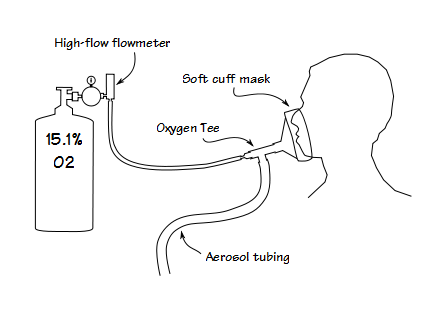
We took an oxygen tee and fit the end opposite the oxygen nipple into the mask. This meant that the HAST gas mixture was blowing towards the mask and thereby maintaining a slight positive pressure inside the mask. If there was a leak around the edge of the mask for any reason the positive pressure would make it less likely for the oxygen concentration to be affected. Even a blow-by system needs some kind of reservoir in order to meet the patient’s inspiratory flow requirements. For this purpose we got a roll of 22 mm aerosol tubing and cut off a 6 foot segment (there’s roughly 10 cc per inch of this kind of tubing, so this makes a reservoir of about 700 cc) and attached it to the remaining limb of the tee. All parts of this breathing circuit are relatively low cost and all parts are disposable.
The final problems with the HAST test were procedural. Some investigators suggest that patients should have their ECG monitored during the HAST test. After some thought we decided this was overkill. The patients that are most likely to become hypoxic enough to have heart arrythmias are those with a low resting SpO2 and are already receiving supplemental O2. The only reason to perform a HAST on these patients is to titrate supplemental O2 and you’d start the test with their normal supplemental O2 flow rate. We also decided not to automatically wait the full 20 minutes before adding supplemental O2. When a patient’s SpO2 drops below 85% we start supplemental O2 at that time.
Finally, some investigators advocate the use of arterial blood gases as the primary way to assess the response to a HAST. We decided that it wasn’t practical to titrate supplemental O2 by ABG and that if we depended on an oximeter to assess titration then it was also accurate enough to assess hypoxia. We also didn’t see any value in performing a HAST just to see if a patient met the criteria for supplemental O2 without being able to immediately start adding supplemental O2 and titrating it as well. Performing an ABG would mean that we’d have to wait for the test results from the chemistry lab before going forward with titration. Patient time and technician time are both valuable and you can only bill for one type of HAST test on a given day so for all these reason we use an oximeter.
Other than the supplies for each test all we had to acquire was a regulator for the HAST gas cylinder and a high-flow flowmeter. This has meant that performing a HAST is relatively low cost for the lab and other than a place to keep the HAST gas cylinder, it requires almost no space. This is important because we do not perform a large number of these tests. Since we started to perform the HAST we have averaged between two and three dozen tests annually. To give some perspective the lab usually has between 6000 and 7000 patient visits annually so at best only around one half a percent of our patients has a HAST. Even though it’s a low volume test doesn’t mean that it isn’t a worthwhile test to perform. As mentioned previously it isn’t possible to accurately predict a patient’s possible hypoxia from a resting ABG with or without spirometry and DLCO results. This means that a HAST is the only way to determine if it is safe for patient with pulmonary disease to travel by airplane and that makes this an important and valuable test to provide to our patients.
References:
Bradi AC, Faughnan ME, Stanbrook MB, Deschenes-Leek E, Chapman KR. Predicting the need for supplemental oxygen during airline flight in patients with chronic pulmonary disease: a comparison of predictive equations and altitude simulation. Can Respir J 2009; 16(4): 119-124.
Dillard TA, Moores LK, Bilello KL, Phillips YY. The preflight evaluation. A comparison of the hypoxia inhalation test with hypobaric exposure. Chest 1995; 107: 352-357.
Dine JC, Kreider ME. Hypoxia altitude simulation test. Chest 2008; 133(4): 1002-1005.
Mohr LC. The hypoxia altitude simulation test. An increasingly performed test for the evaluation of patients prior to air travel. Chest 2008; 133(4): 839-842.
Robson AG, Hartung TK, Innes JA. Laboratory assessment of fitness to fly in patients with lung disease: a practical approach. Eur Respir J 2000; 16: 214-219.
Seccombe LM, Kelly PT, Wong CK, Rogers PG, Lim S, Peters MJ. Effect of simulated commercial flight on oxygenation in patients with interstitial lung disease and chronic pulmonary disease. Thorax 2004; 59: 966-970.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


Hi,
Really interesting post. Our lab currently uses a very very simple technique (maybe too simple?!). We use a Venturi mask with 100% Nitrogen flowing through and just monitor SpO2 throughout. The mask isnt a very good fit as they are single use so i suspect there may be a lot of leaking…?
We have a lot of problems testing paediatric patients as well. They find it difficult to sit still for 20 minutes! Would be interested to hear your thoughts.
Thanks
Rachel –
I’m not a big fan of Ventimasks since they are not terribly accurate but they do have the advantage of only needing compressed nitrogen. If you’re going to use them you should check the output with an oxygen analyzer. Leaks shouldn’t be an issue since the combined flow rate of the nitrogen and entrained air should be enough to meet the patient’s needs and in fact because ventimasks are sensitive to back-pressure they shouldn’t be tight fitting anyway. Pediatric patients? How about giving them a computer game they can play while taking the test?
I haven’t priced ventimasks for a while but I have to wonder if the money you save using inexpensive nitrogen rather than a more expensive gas mix is being lost in paying for the ventimasks.
Regards, Richard
Which fitting did you use to get 15% FIO2? How was the calculation made.
Thanks!
Emin –
We have a tank of 15% O2 that we use for HAST tests.
Regards, Richard
Hi Richard
most informative as usual. We use the body box method and simply feed the tubing for the oxygen and the nitrogen through the rubber seal on the side of the box door. Not airtight but that doesn’t seem to matter. I concur that you need a lot of gas to get the box atmosphere to the correct level. And of course an oxygen analyser and oximeter. It does however confer the advantage of easily titrating oxygen (if required). In addition, we test test many neonates with CLD – the mother holds the child while sitting in the box. This technique makes it much easier to manage small children.
Regards
Paul
Paul –
Just curious but how long does it take to get the box to the right FIO2? I assume the oxygen tubing is for a nasal cannula (for titrating supplemental O2) so does that mean you just add nitrogen? How stable are you able to keep the FIO2 and is there any CO2 buildup?
– Richard
Hi Richard, time will vary a little depending on the size of the subject in the box. Geneally 15-20 minutes would be typical. FiO2 is surprisingly easy to control and once we reach the desired FiO2 we turn the nitrogen supply off and monitor the patient. I guess the large volume of gas is less sensitive the change due to tidal breathing. CO2 build up can be an issue but doesnt seem to have had any effect on the clients. In comparison I recently performed a HAST with a HR valve connected to a 2L anaesthetic bag via CPAP tubing. Nitrogen and air were fed via a Y piece into the bag. Very effective but very sensitive and I found it much harder to stabilize the FiO2. But the client was wheelchair bound and couldnt sit in the box so we needed an alternative. Thanks again for the posts
regards
Paul
Hello Richard!
I love your blog thank you for having this resource available to share your expertise!
My question is that currently we use a tight fitting CPAP mask and deliver 15% FiO2 using a premixed tank. We then titrate O2 to maintain SPO2 above 90% using a NC. My question is that does using the NC build a reservoir or skew results in any way? We also want to perform this on infants/neonates with the same method.
Is it more accurate to use a blender or 15% tank with NC and why?
Thank you for your advice!!
Emin –
It depends on the flowrate to the mask. We use 15 LPM blowing at the mask and are reasonably certain there’s no O2 buildup. Blenders are only accurate to a few percent at best unless you can use a good O2 analyzer to verify the percent. The problem is that unless it’s a real good O2 analyzer it too is only accurate to a couple percent. I believe a pre-mixed tank is still the best way to go.
– Richard