I have been searching through Pulmonary Function videos on YouTube in order to find ones I thought would be useful for technician education. So far what I’ve found have been intended either for medical students or for patients and not, in my opinion, particularly suitable for training technicians. Lately I’ve been looking at videos about lung volumes and have seen a half dozen presenters describe lung volume subdivisions using the same graph we’ve come to know and love with varying degrees of effectiveness and obfuscation.
In a discussion of helium dilution lung volume measurements one of the presenters made an interesting statement and that was that “helium does not pass the alveolar-capillary barrier which means it stays inside the lungs during the test”. This is wrong on multiple levels. First, the alveolar-capillary membrane evolved for gas exchange and does not discriminate against individual gases so there is no barrier. Second, the reason that gases can be used as tracer gases or as probes of pulmonary circulation has entirely to do with gas solubility. Third, since it was a university-sponsored video with other egregious errors (for example did you know that lung volumes are measured in ml/kg?) what the heck are they teaching their medical students?
Gases can and will be absorbed by blood and tissue. The quantity of gas that can be absorbed is determined by the gas’s solubility and the Bunsen solubility coefficient is a measure of how much gas is absorbed (usually in milliliters of gas per milliliter of liquid) when the gas is at 1 atmosphere of pressure. When there is a multi-gas mixture, the quantity of gas absorbed for individual gas is calculated by:
The Bunsen Solubility of the common respiratory gases are as follows:
| Gas: | Solubility in Blood: |
| Acetylene (C2H2) | 0.739 |
| Carbon Dioxide | 0.515 |
| Carbon Monoxide | 0.0189 |
| Dimethylether | 9.0 |
| Freon-22 | 0.673 |
| Helium | 0.0094 |
| Methane | 0.0468 |
| Nitrogen | 0.012 |
| Nitrous Oxide (N2O) | 0.412 |
The reason that helium (and to some extent methane) are useful as tracer gases is because of their low solubility. During a helium dilution FRC test, the helium concentration in the testing circuit usually starts around 7% plus or minus a bit. Since the normal human blood volume is around 5 liters, that means that the amount of helium absorbed into a subject’s blood during the test is going to be no more than:
or 3.29 ml.
Some helium will also be absorbed by the lung itself but that will be an even smaller amount, so at a rough estimate, approximately 5 ml of helium will be absorbed during the test. That’s not zero, but it’s also less than 1 percent of the total amount of helium in the test gas circuit. It’s not that helium doesn’t pass the alveolar-capillary “barrier” as the presenter stated in the video but because it is so insoluble only a miniscule amount is absorbed. A proper FRC calculation will take this into account but even if it is ignored the amount of error will be negligible and probably less than the error bars for the helium analyzer and spirometer volume.
Methane has replaced helium for most single-breath DLCO tests. It’s more soluble than helium, but not by that much. Calculating the absorption of methane during a DLCO test is more complicated because it occurs over a shorter time period but since a normal resting cardiac output is roughly 5 liters/min and the breath-holding time for a DLCO test is nominally 10 seconds, that means that the amount of blood exposed to DLCO gas mixture is approximately 833 ml. The amount of methane absorbed is therefore:
or 0.116 ml, which is very approximately 0.7% of the total amount of methane inhaled. Again not zero, and again it should be accounted for, but again also negligible and likely less than the error bar of the gas analyzer.
Nitrogen is also a relatively insoluble gas (although that doesn’t mean that its solubility isn’t a consideration for divers and hyperbaric chambers). During a nitrogen washout lung volume test, a subject replaces the nitrogen in their lung with oxygen. The total volume of nitrogen in the blood is approximately:
or about 47 ml, which is about 1.2% of the total volume of nitrogen in the lung and is about the same level of error as for helium and methane measurements. Admittedly this does not account for the nitrogen in the muscles and tissue of the body, but these stores are only slowly depleted and for the short time interval involved in a nitrogen washout test they don’t make a particularly great contribution to exhaled nitrogen.
Gases with high solubilities on the other hand, have been used to measure cardiac output. Acetylene, Dimethylether (DME) and Freon-22 have been used for this purpose. The calculations are a bit more involved but basically since the amount of soluble gas in an inhaled gas mixture is known, its solubility and the rate at which it disappears over time can be used to measure pulmonary blood flow. Specifically in a single-breath maneuver:
Where:
Qc = pulmonary capillary blood flow rate
Solb = solubility coefficient of gas in blood
Solt = solubility coefficient of gas in lung tissue
Vt = volume of lung tissue
Fa = fractional concentration of soluble gas in alveolar air
t1, t2 = starting and ending time of breath hold
Finally, the solubility of oxygen in blood plasma is why hemoglobin is necessary. Oxygen is actually poorly soluble. The oxygen content of blood is calculated from:
For a PaO2 of 100, without hemoglobin the amount of oxygen in a decaliter of blood would only be about 0.31 ml. With hemoglobin it is about 20 ml and that difference is what makes it possible for us to exist (or at least able to walk and talk, since without hemoglobin we wouldn’t have the energy to be much more than lumps of protoplasm that were unable to move or think more than slightly).
The differences in the solubility of gases allows us to make a number of interesting physiological measurements. Gas solubility may be a slightly esoteric aspect of physics, chemistry and pulmonary function testing and it is true that calculations with a low degree of error can be made without accounting for its effects, but that doesn’t mean it doesn’t matter and that it shouldn’t be taught correctly even if only to medical students.
References:
Brusasco V, Crapo R, Viegi G et al. ATS/ERS Task Force: Standardisation of lung function testing. Standardisation of the measurements of lung volumes. Eur Respir J 2005; 26: 511-522.
Cander L. Solubility of inert gases in human lung tissue. J Applied Physiol 1959; 14(4): 538-540.
Ramage JE, Coleman RE, MacIntyre NR. Rest and exercise cardiac output and diffusing capacity assessed by a single slow exhalation of methane, acetylene and carbon monoxide. Chest 1987; 92: 44-50.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.



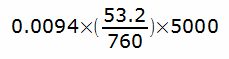
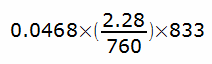
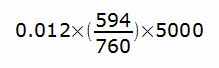
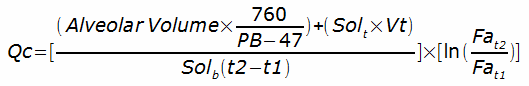
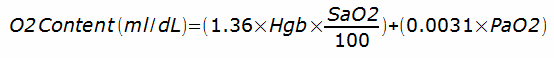
Thank you for explaining that bit about the Helium. I also wondered how Helium miraculously does not diffuse through the alveolar-capillary barrier, simply because it is ‘inert’. Very clear explanation and skipped in most outher sources.