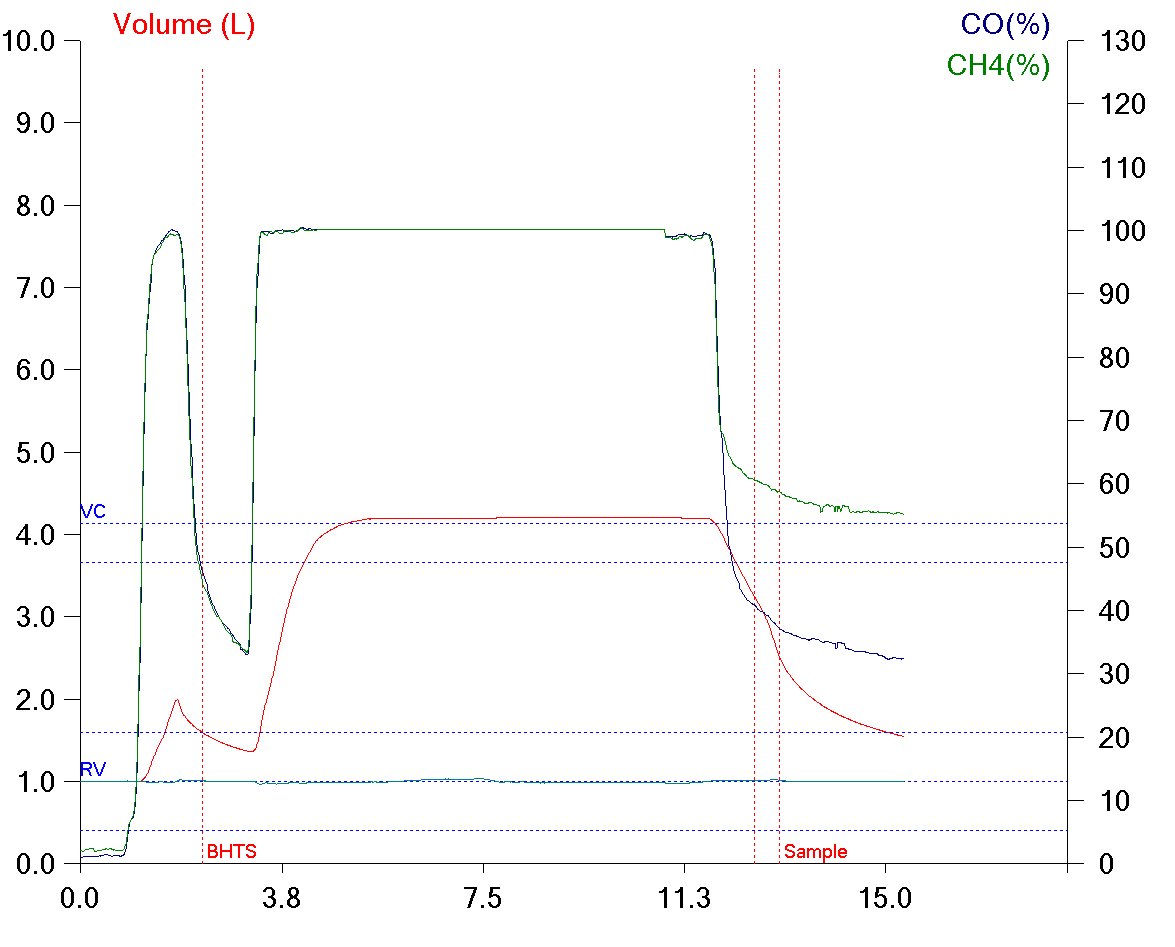
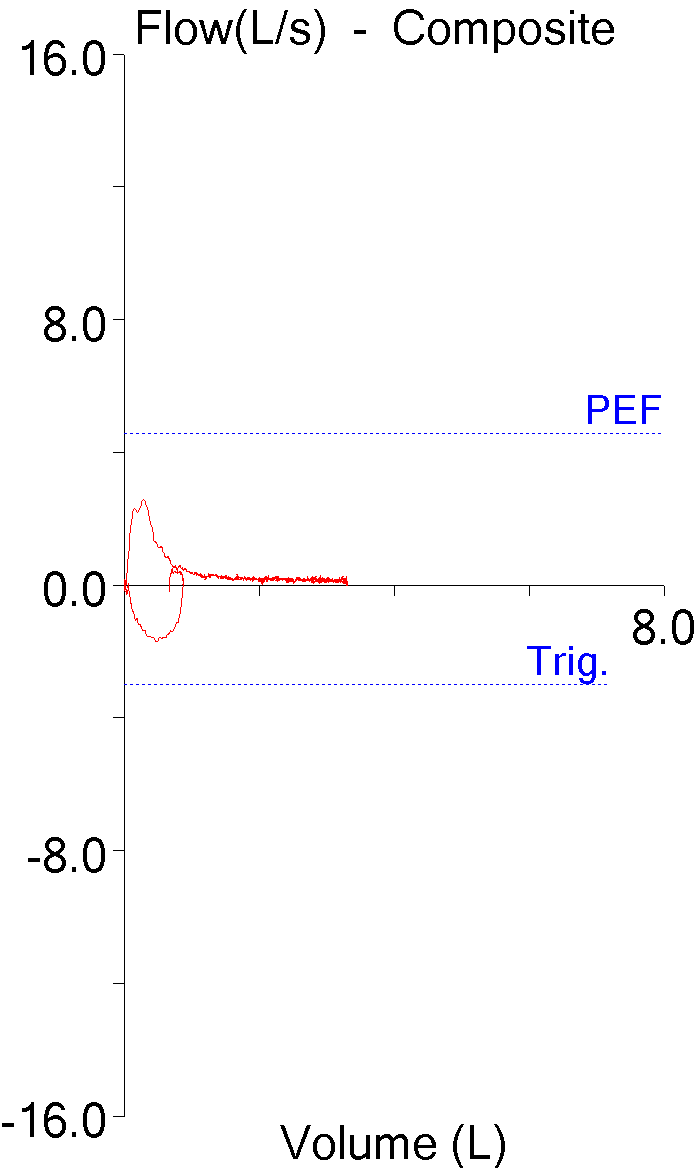
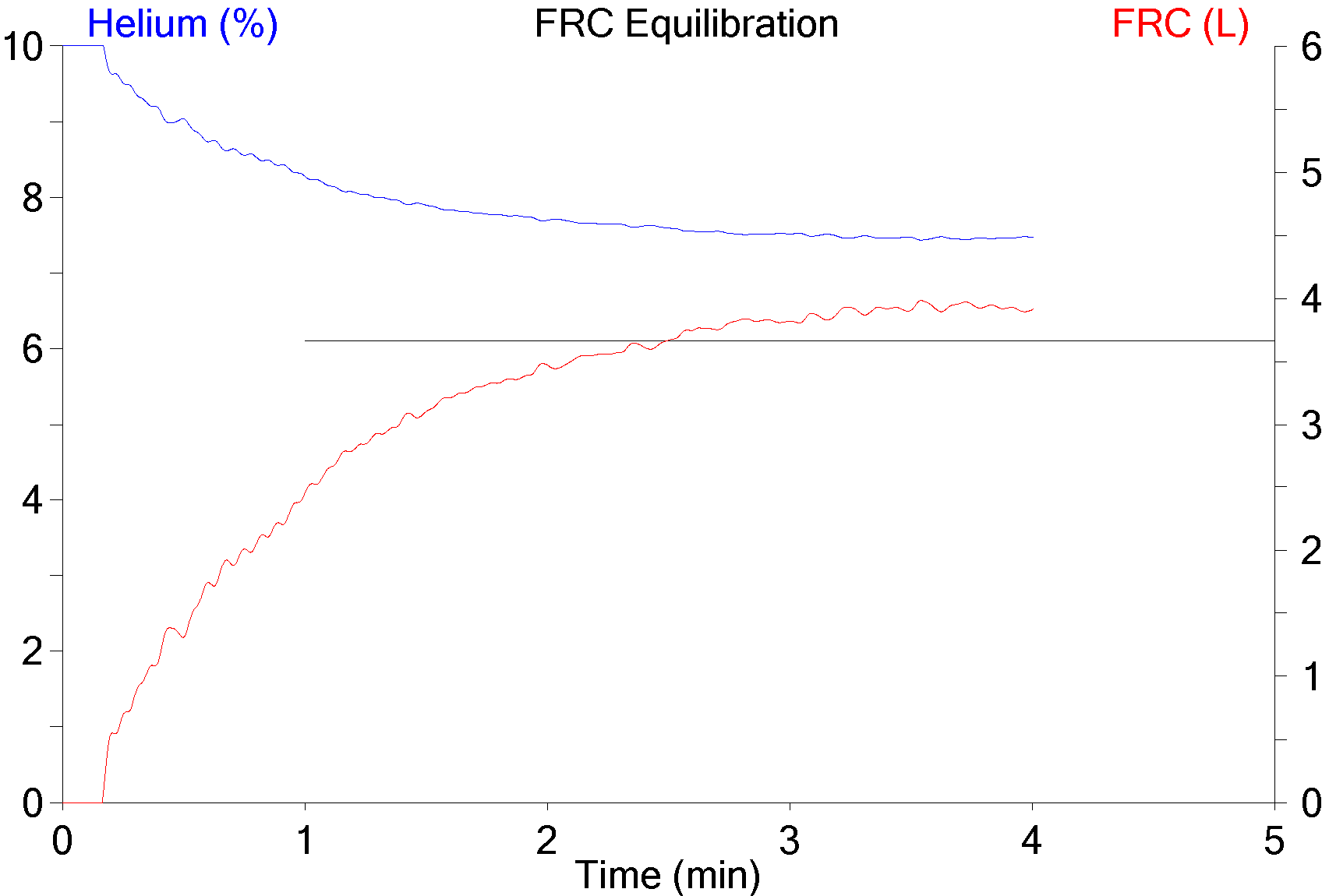
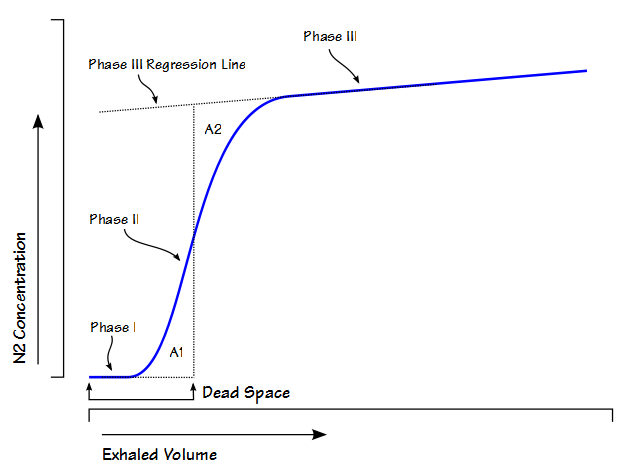
While reading a recently published article I found they had performed response to hypoxia and hyperoxia testing as part of the study. At one time or another in the past I’ve read about response to hypoxia testing but I’d never heard about hyperoxia testing before. I had some difficulty understanding their interpretation of the study’s results and for this reason I’ve spent some time reading up on the subject. I’m not sure this helped because there appears to be a lack of consensus not in only how to perform these tests but also in how they are interpreted, except perhaps in the most simplistic sense. Hypoxia and hyperoxia testing has been performed primarily to gain a deeper understanding of the way in which the peripheral (carotid) and central chemoreceptors function. There are a variety of sensor-feedback network models and results are often presented in terms of one model or another and this makes comparing results from different studies difficult. Interpretation and comparison is further complicated by the fact that results depend not only on the length of time that hypoxia or hyperoxia is maintained but whether the subject was exposed to hypoxia, hyperoxia or hypercapnia previously.
The ventilatory response to hypoxia tends to have three phases. First, once a subject begins breathing a hypoxic gas mixture within several seconds there is a rapid increase in minute ventilation known as the Acute Hypoxic Ventilatory Response (AHVR). Second, after several minutes there is a decrease in ventilation and this is usually called the Hypoxic Ventilatory Depression (HVD). Third, there is a progressive rise in ventilation after several hours which is related to acclimatization to altitude. It is the first phase, AHVR, that is most commonly measured during a hypoxic ventilatory response test. The actual length of time that is spent in any of these phases is widely variable between individuals and there is also a relatively large day-to-day variability within the same individual.
Continue reading