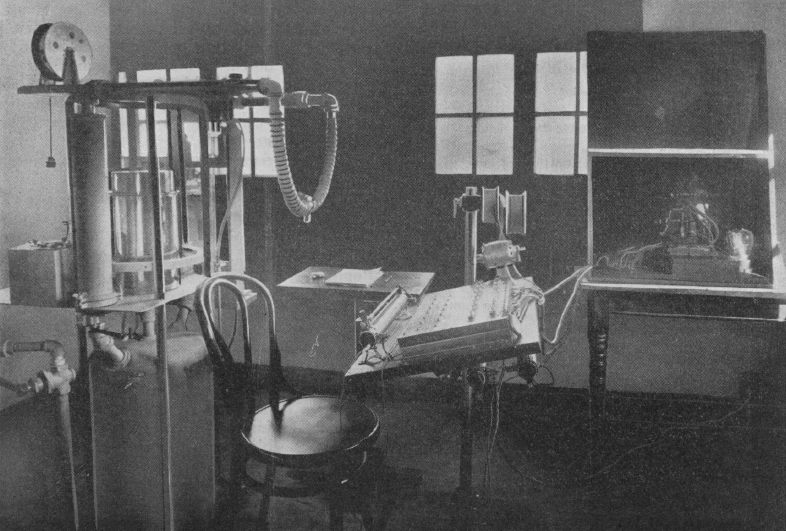
From: Manual of Practical Physiology: Designed for the Physiological Laboratory Course in the Curriculum of the American Association of Medical Colleges, By John Conrad Hemmeter, 1912, page 117. An actual photograph instead of a drawing!
Spirometer, 1915
Spirometer, Henderson Rebreather Apparatus, 1920
From: The examination and classification of aviators with special reference to the effects of high altitiude. By JF Grant, California State Journal of Medicine, 1920, page 99.
“The Henderson Rebreathing Apparatus has been perfected for such tests [low O2 at altitude]. During the test the subject breathes the air in the tank. He sits with a clip placed on the nose and comfortably adjusted mouthpiece in the mouth, which is suitably connected by means of inch rubber tubing with light automatic valves. He inhales the air through the respiratory valve and exhales through the expiratory valve into a cartridge containing an absorbant for carbon dioxide , namely sodium hydroxide in cake form. The exhaled air is thus freed from carbon dioxide as it is returned to the tank. A spirometer compensates for changes in volume and writes a record of the respiration on the revolving drum of a kymograph. By this arrangement the subject continues to rebreathe the air in the tank, from which he gradually absorbs oxygen. As the percentage of oxygen decreases, the subject, in effect physiologically, is slowly ascending to higher altitudes. The volume of air rebreathed is sufficient to require between 25 and 30 minutes to lower the amount of oxygen to 8 or 7 percent, which is equivalent to altitudes of 25,000 to 28,000 feet.”
Spirometer, Henderson Rebreather Apparatus, 1920
From: The examination and classification of aviators with special reference to the effects of high altitiude. By JF Grant, California State Journal of Medicine, 1920, page 99.
“The Henderson Rebreathing Apparatus has been perfected for such tests [low O2 at altitude]. During the test the subject breathes the air in the tank. He sits with a clip placed on the nose and comfortably adjusted mouthpiece in the mouth, which is suitably connected by means of inch rubber tubing with light automatic valves. He inhales the air through the respiratory valve and exhales through the expiratory valve into a cartridge containing an absorbant for carbon dioxide , namely sodium hydroxide in cake form. The exhaled air is thus freed from carbon dioxide as it is returned to the tank. A spirometer compensates for changes in volume and writes a record of the respiration on the revolving drum of a kymograph. By this arrangement the subject continues to rebreathe the air in the tank, from which he gradually absorbs oxygen. As the percentage of oxygen decreases, the subject, in effect physiologically, is slowly ascending to higher altitudes. The volume of air rebreathed is sufficient to require between 25 and 30 minutes to lower the amount of oxygen to 8 or 7 percent, which is equivalent to altitudes of 25,000 to 28,000 feet.”
Spirometer, Gas Meter Calibration, 1920
From: The calibration and accuracy of gas meters. By August Krogh. Biochemical Journal, 1920, Chapter XXIX, page 19.
“The calibration spirometer (fig 1) is an adaptation of a similar instrument employed by the Dannsk Maalerfabrik, the chief improvement being that I have arranged an automatic record of the volume per revolution of the meter calibrated. The drum (1) which is as nearly as possible cylindrical is suspended by a band of soft steel and counterbalanced by the adjustable weight (2). It moves up and down with a minimum of friction. When the drum is in its highest position the weight can be held by an electromagnet (not shown in the figure) from which it can be released at any desired moment by breaking the current. Along the steel band a fine brass wire is arranged carrying a number of lead weights (3). These can be displaced along the wire and a complete equilibrium in all positions of the drum can thus be secured. When the weight (2) is reduced, any desired pressure up to 20 mm. water can be produced in the spirometer. The revolving fan (4) which can be driven from outside is used for mixing purposes. In calibration work with air it is revolved for a few minutes when the spirometer has been filled, to secure a uniform temperature and perfect saturation of the air with water vapour.
“The drum is painted inside and out with a hydrophobe compound so that its volume will not be altered by water absorbed to its wall. This detail is very important. When left in contact with water, ordinary paint will take up a considerable volume of water which cannot drain off. According to experiments a painted tinplate continued to gain in weight while immersed in water for a couple of months, ultimately to the extent of 2.5 gm per 100 sq. cm. Such an increase would in my spirometer diminish by 3.5 cc the effective volume per cm.
“The spirometer drum has been calibrated by measuring a large number of internal diameters (133) evenly distributed over the whole length and circumference of the drum. These measurements have given as the average diameter 450..135 +/- 0.09 mm or a volume of 1591.4 cc per cm. height. When the drum is used in the spirometer a lowering of 1 cm. will drive out a little more air than the corresponding volume of the drum because the surface of the water in the reservoir will be raised when 1 cm. of wall of the the drum is lowered into it. The volume of 1 cm. of the wall of the drum is 9.4 cc. The rise in the water level will of, course, correspond to 9.4 cm., but since only 19.0% of the water surface is inside the drum the extra volume corresponding to 1 cm. will be 19/100 x 9.4 = 1.8 cc and the effective volume of 1 cm. is therefore 1593.2 cc. A further correction for the volume of the hydrophobic paint and for water adhering to the inside reduced the volume to 1593.0 cc. This latter correction is of course a little arbitrary. When the drum has been rapidly raised the water must be allowed to drain off for about 5 minutes before a practically constant volume is attained. The water adhering to it at first may amount to a couple of cc. per cm. or more.”