We recently performed a 6-minute walk test with helium-oxygen (heliox) for a patient of one of the physicians that specializes in airway stenting. His reasons for the test weren’t particularly clear (and he hasn’t bothered to try to clarify them with me) but most probably it has to do with differentiating between central and peripheral airway obstruction. Interestingly, he predicted the patient would have a significant improvement in 6-minute walk distance and instead there was little difference between the heliox 6MWT and one performed with 3 LPM supplemental O2.
| 6MWT: | SaO2: | Distance: |
| 80% Helium – 20% O2, by mask | 95% | 440 meters |
| 3 LPM O2, by nasal cannula | 98% | 457 meters |
Helium is an inert, insoluble, low mass gas and both its therapeutic use and its use in physiological measurements has to do with it’s low density (and the fact that it’s highly insoluble, but that’s for purposes different than those discussed here).
| Density (g/m3) | |
| He | 0.179 |
| N2 | 1.251 |
| O2 | 1.429 |
| Air (78% N2, 21% O2) | 1.293 |
| Heliox (80% He, 20% O2) | 0.429 |
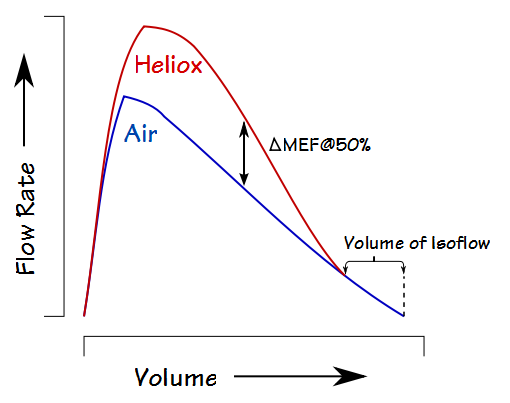
A typical way to assess its effect is by comparing air and heliox flow-volume loops:
Interestingly, despite an apparent increase in flow rates there is usually no significant difference in FEV1 (one study showed a range of +2% to +7% in a group of over 1500 subjects). The most common heliox FVL measurements are the change in expiratory flow at 50% of the FVC (ΔMEF@50%) and the Volume of Isoflow (which is the point at which the air and heliox expiratory flows become equivalent). Many of the earlier studies with heliox also measured ∆MEF@75% and ∆FEF25-75, and a tiny handful of studies (particularly given the technical difficulties) have measured ∆RAW and ∆sGAW.
The increase in expiratory flows while breathing heliox and the lung volume at which they occur has been traditionally explained in terms of turbulent flow, laminar flow and the equal pressure points (EPP) of the airways. Specifically, the maximal expiratory flow at any given lung volume varies directly with the transpulmonary pressure (from elastic recoil and contraction of the respiratory muscles) and inversely with airway resistance between the alveoli and the EPP. The EPP is where the lateral airway wall pressure is equal to the pressure in the surrounding lung tissue. During a forced exhalation in normal subjects the EPP remains in the large airways until about 75% of the FVC has been exhaled.
The resistance to airflow in the large airways is primarily due to convective acceleration (the increase in gas velocity due to decreasing cross-sectional area as exhaled air moves from smaller to larger airways) and turbulence, both of which are dependent on gas density. Flow changes from laminar to turbulent as its velocity increases and this is described the Reynolds number:
Flows are generally laminar at a Reynolds number less than 2000, fully turbulent above 2800 and transitional inbetween. The Reynolds formula shows that because of heliox’s lower density at the same velocity turbulence will be reduced and a recent study estimated that it is reduced up to 50%. Once flow is turbulent however, density continues to be a primary factor and velocity is determined by:
Laminar flow predominates in the smaller airways (<2.0 mm in diameter) and laminar flow is dependent on gas viscosity and not gas density:
| Viscosity (10-5 Pa s) | |
| He | 1.87 |
| N2 | 1.66 |
| O2 | 1.95 |
| Air (78% N2, 21% O2) | 1.73 |
| Heliox (80% He, 20% O2) | 1.89 |
The important point is that turbulent flow is density-dependent and viscosity-independent, and laminar flow is viscosity-dependent and density-independent.
Because disorders like asthma, emphysema and chronic bronchitis narrow the smaller airways (although of course from different processes) and for these disorders this is where the primary resistance to exhalation resides. This means that the difference between heliox-breathing and air-breathing flow-volume loops tends to be small for these disorders.
One study showed that in a group of subjects with chronic asthma, those that responded to a bronchodilator showed an increase in flow rates on their flow-volume loops similar to normal subjects whereas those subjects who did not have a bronchodilator response showed no significant change in their flow-volume loops. Moreover, the BD-responders showed a decrease in airway resistance (RAW) while on heliox and non-responders did not.
This very neat explanation has been called into question by a couple of recent papers that showed (at least mathematically) that despite the fact that laminar flow is primarily a visosity-related function that gases undergo a lateral acceleration (i.e. the direction of airflow changes) as they pass airway bifurcations and that the lower density of heliox improves airflow under these circumstances. If true this would imply that there should be improvements in expiratory flow rates regardless of whether the flow is laminar or whether it is turbulent.
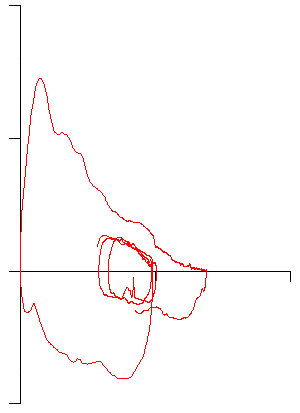
So why didn’t our patient’s 6-minute walk distance improve? Their recent PFT results were low normal but still within normal limits:
| Observed: | %Predicted: | |
| FVC: | 2.78 | 82% |
| FEV1: | 2.12 | 82% |
| FEV1/FVC: | 76 | 99% |
| TLC: | 4.45 | 83% |
| DLCO: | 15.78 | 83% |
And given their age (66) there’s nothing particularly remarkable about their flow-volume loop:
Interestingly, there is very little literature on exercise and heliox in normal subjects. One article performed constant-workload testing of a small group of young subjects and showed no significant change in VO2 or minute ventilation although it did show that the work of breathing decreased with heliox. Another study showed an increase in minute ventilation with heliox in an older cohort but unfortunately did not measure gas exchange.
The majority of research on heliox and exercise has been on subjects with pulmonary disease. One relatively pertinent study of 6MWT testing on subjects with COPD showed that the heliox 6-minute walk distance was significantly higher than when performed on room air, nasal O2 or O2 by mask. This is generally seconded by a review article that closely analyzed eight studies of COPD and exercise and concluded that heliox improved exercise capacity and decreased dyspnea.
There is however, equivocal and sometimes contradictory evidence about why exercise capacity improves in COPD. Although one study on COPD and exercise with heliox showed no change in IC and hyperinflation others had exactly the opposite results. It’s been suggested that heliox improves CO2 elimination (CO2 diffuses many times faster in heliox than in air) and a number of studies have shown small but statistically significant decrease in PaCO2 while breathing heliox and one study showed a small improvement in Ve/VCO2. Regardless of the cause however, most studies showed a decrease in dyspnea and an increase in endurance time when patients with COPD exercised while breathing heliox.
None of this gives any physiological reason why our patient did not improve their 6-minute walk distance and I’m left with other factors as being more likely. I’m particularly curious that the patient said they had much more significant dyspnea and fatigue while breathing heliox than while on supplemental O2. This is somewhat contradictory to the literature that tends to indicate that subjects have a decrease in dyspnea with heliox (I’m also curious why the physician ordered the comparison 6MWT be performed with supplemental O2, particularly given the patient’s normal DLCO).
During the 6-minute walk the heliox and oxygen tanks were wheeled by a staff member and I have to wonder if this somehow set the pace for the patient. This is hard to verify since I did not witness the test being performed but the staff that were there said they stayed behind and the patient set the pace. This means it could be a musculo-skeletal or motivation issue but at the moment that has to remain speculative.
Given that heliox testing can be at least somewhat physiologically informative, why don’t we perform this kind of testing more often?
One reason is that performing spirometry with heliox is technically difficult. Pneumotachs are sensitive to gas density and must be calibrated using a heliox mixture. Given the difference in thermal conductivity and the speed of sound in heliox this limitation may also apply to mass flow sensors (hot wire anemometers) and ultrasonic spirometers but I’ve been unable to find any study that either verified this or attempted to use either of these devices. Although volume displacement spirometers are insensitive to gas composition (and this is one area where they have a significant advantage over flow sensors) they have become relative rare.
There is also a lack of standardization about how to prepare for the test. Some researchers had their subjects wash out the resident nitrogen in their lung by breathing heliox for 2 to 10 minutes before performing spirometry. Other (most) researchers have used 3 vital capacity breaths of heliox instead and at least one study showed no difference in ΔMEF@50% and the volume of isoflow between the two approaches in normal subjects. For this reason the 3-breath approach is probably reasonably acceptable for subjects with normal lungs but in subjects with airway obstruction and ventilation inhomogeneities it remains questionable.
Finally though, the real reason is that heliox testing lacks clinical relevance and this is largely due both to a lack of sensitivity and to testing variability. When it was first performed in the 1970’s the volume of isoflow in particular was thought to be able to detect obstruction in the small airways of smokers and individuals exposed to pollutants (aka small airways disease) but within a decade several studies showed little correlation between ΔMEF@50%, the volume of isoflow, the FEV1, and symptoms or exposure history. At the same time several studies have shown that the test-to-test variability for these values within the same individual is more or less the same magnitude as it is between normal subjects and those with “small airways disease”.
One reason for this is that the measurements depend on the FVC volume and one study of over 1500 subjects found that only about half were able to perform a heliox FVC that was within 5% of an air FVC. Interestingly and somewhat counter-intuitively, FVC’s performed with room air were far more often to be larger than those performed with helox. The same studies also showed that the test-to-test variability for the volume of isoflow is exceptionally high even when FVC reproducibility was controlled and that this variance did not improve with experience.
Heliox has been shown to be therapeutically useful in asthma, COPD, upper airway disorders, airway tumors, vocal cord dysfunction and a variety of other problems. Although testing with heliox can give some indication about whether the majority of an individual’s airway resistance resides in the larger or smaller airways, the results from this testing are often unrepeatable and for these reasons the usefulness of heliox will likely remain on the therapeutic side of things.
References:
Ashutosh K, Mead G, Dickey JC, Berman P, Kuppinger M. Density dependence of expiratory flow and bronchodilator response in asthma. Chest 1980; 77(1): 68-75
Babb TG, DeLorey DS, Wyrick BL. Ventilatory response to exercise in aged runners breathing He-O2 or inspired CO2. J Appl Physiol 2003; 94: 685-693.
Berend N, Nelson NA, Rutland J, Marlin GE, Woolcock AJ. The maximum expiratory flow-volume curve with air and a low-density gas mixture. An analysis of subject and observer variability. Chest 1981; 80(1): 23-30.
Despas PJ, Leroux M, Macklem, PT. Site of airway obstruction in asthma as determined by measuring maximal expiratory flow breathing air and a helium-oxygen mixture. J Clin Invest 1972; 51: 3235-3242.
Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC, Sheel AW. Exercise-induced arterial hypoxemia and the mechanics of breathing in healthy young women. J Physiol 2013: 591(12): 3017-3034.
Dosman JA, Chong P, Cotton DJ. Detection of peripheral airways obstruction in smokers using air vs helium spirometry. Bull Eur Physiopath Resp 1978; 14: 137-143.
Fairshter RD, Novey HS, Wilson AF. Site and duration of bronchodilation in asthmatic patients after oral administration of terbutaline. Chest 1981; 79(1); 50-57.
Gelb AF, Klein E. The volume of isoflow and increase in maximal flow at 50 percent of forced vital capacity during helium-oxygen breathing as tests of small airway dysfunction. Chest 1977; 71(3): 396-399.
Hess DR, Fink JB, Venkataraman ST, Kim IK, Meyers TR, Tano BD. The history and physics of heliox. Respir Care 2006; 51(6): 608-612.
Hunt T, Williams MT, Frith P, Schembri D. Heliox, dyspnoea and exercise in COPD> Eur Respir Rev 2010; 19: 115, 30-38.
Katz IM, Martin AR, Muller P-A, Terzibachi K, Feng C-H, Caillibotte G, Sandeau J, Texereau J. The ventilation distribution of helium-oxygen mixture and the role of inertial losses in the presence of heterogeneous airway obstructions. J Biomechanics 2011; 44: 1137-1143.
Knudson RJ, Bloo, JW, Kaltenborn WT, Burrows B, Lebowitz MD. Assessment of air vs helium-oxygen flow-volume curves as an epidemiological screeing test. Chest 1984; 86(3): 419-423.
Lam S, Abboud RT, Chan-Yeung M, Tan F. Use of maximal expiratory flow-volume curves in air and helium-oxygen in the detection of ventilatory abnormalities in population surveys. Am Rev Respir Dis 1981; 123: 234-237.
MacDonald JB, Cole TJ. The flow-volume loop: reproducibility of air and helium-based tests in normal subjects. Thorax 1980; 35: 64-69.
Marciniuk DD, Butcher SJ, Reid JK, MacDonald GF, Eves ND, Clemens R, Jones RL. The effects of helium-hyperoxia on 6-minute walking distance in COPD: A randomized controlled trial. Chest 2007; 131(6): 1659-1665.
Palange P, Valli G, Onorati P, Anonucci R, Paolette P, Rosato A, Manfredi F, Serra P. Effect of heliox on lung dynamic hyperinflation, dypnea and exercise endurance capacity in COPD patients. J Appl Physiol 2004; 97: 1637-1642.
Pecchiari M, Pelucchi A, D’Angelo E, Foresi A, Milic-Emili J, D’Angelo E. Effect of heliox breathing on dynamic hyperinflation in COPD patients. Chest 2004; 125: 2075-2082.
Rudnow M, Hill AB. Helium-Oxygen mixtures in airway obstruction due to thyroid carcinoma. Can Anaesth Soc J 1986; 33: 498-501.
Skrinkas, GJ, Hyland RH, Hutcheon MA. Using helium-oxygen mixtures in the management of acute upper airway obstruction. Can Med Assoc J 1983; 128: 555-558.
Swidwa DM, Montenegro HD, Goldman MD, Lutchen KR, Saidel GM. Helium-Oxygen breathing in severe chronic obstructive pulmonaory disease. Chest 1985; 87(6): 790-795.
Zeck RT, Solliday NH, Celic L, Cugell DW. Variability of the volume of isoflow. Chest 1981; 79(3): 269-272.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License