My hospital has an active liver transplantation program and all transplant candidates get a full panel of PFTs in my lab. The number of liver transplant candidates we get varies from week to week but probably averages between 150 and 200 a year. As with any population a certain number of them have COPD or other lung diseases but there are some that have normal spirometry and lung volumes but a reduced DLCO. This latter group of patients likely have hepatopulmonary syndrome (HPS).
There are three hallmarks of hepatopulmonary syndrome. First is the presence of a liver disease (most commonly cirrhosis and hepatitis although liver cancer can also be a cause). Second are intrapulmonary vascular dilations (usually determined by transthoracic contrast-enhanced echocardiography). The third are gas exchange abnormalities, which include hypoxia and a reduced DLCO. The more severe cases of HPS may also have some additional (and somewhat unusual) symptoms: platypnea (dyspnea induced by the upright position) and orthodeoxia (a decrease in PaO2 and SaO2 when changing from the supine to upright positions).
For reasons that aren’t completely clear liver disease can cause chronic vasodilation of the systemic and pulmonary vasculature. The normal diameter of the pulmonary capillaries is in the range of 8-15 microns. When dilated due to liver disease they can be as large as 100 or even 500 microns in diameter. This allows mixed-venous blood to pass through the pulmonary capillaries very quickly or even directly into the pulmonary veins, and this in turn causes arterial hypoxia.
HPS severity is usually graded according to the level of hypoxia. First, for HPS to be considered at all, an individual’s alveolar-arterial oxygen gradient (PAaO2) needs to be greater than 15 mm hg. After that HPS is graded using PaO2 (room air, sea level) as:
| PaO2 (mm Hg) | |
| Mild | ≥80 |
| Moderate | <80, ≥60 |
| Severe | <60, ≥50 |
| Very Severe | <50 |
Interestingly CO2 retention is never seen in hepatopulmonary syndrome, and in fact since these individuals usually chronically hyperventilate, hypocapnia (PaCO2 < 35) and respiratory alkalosis are often present.
The reasons that DLCO is reduced in HPS are complex and one study or another has indicated that it is due in part to:
- Increased V/Q mismatching
- Increased intrapulmonary shunting
- Increased thickness of the alveolar-capillary membrane
- An increased distance between the alveolar membrane and the central stream of the blood flow through the dilated capillaries doesn’t allow for adequate diffusion of carbon monoxide
- A decreased pulmonary capillary transit time
Although the pulmonary capillary dilation is usually seen diffusely throughout the lung, on a local level it is often heterogeneous. This causes increased V/Q mismatching and is often exacerbated because the ability of the pulmonary vasculature to vasoconstrict due to hypoxia is blunted or even absent. This causes poorly ventilated lung units to continue to be perfused and an increase in intrapulmonary shunting.
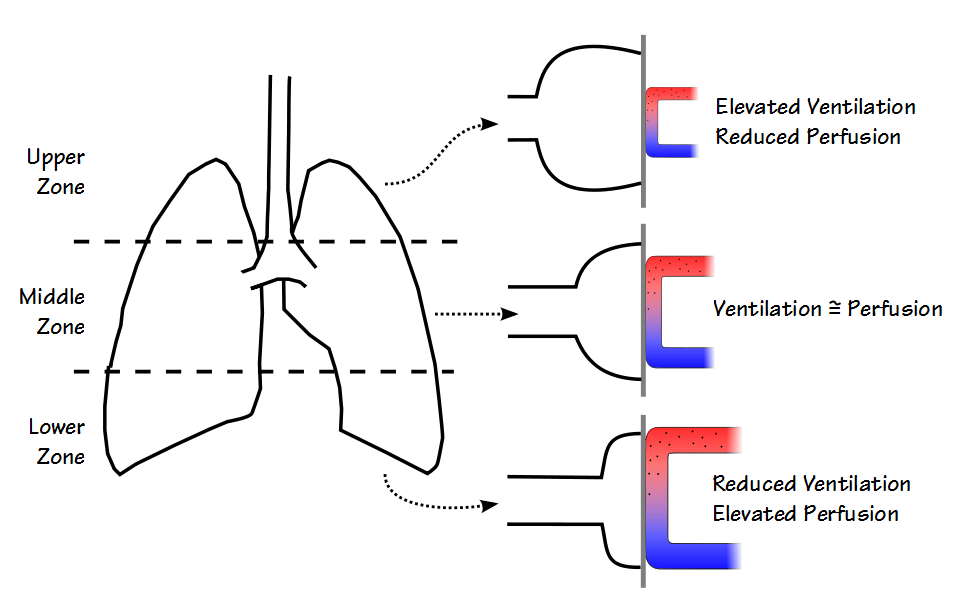
In addition the relationship between ventilation and perfusion within the lung is affected by gravity. In the early 1960’s J. B. West proposed a three zone lung model.
In this model the upper zone of the lung has the lowest pulmonary arterial pressure and therefore the lowest perfusion. At rest it therefore has the highest ventilation/perfusion ratio. The lower zone has the highest pulmonary arterial pressure, the highest perfusion and at rest the lowest V/Q ratio. Ventilation and perfusion tend to be relatively well matched in the middle zone.
The more severe forms of HPS have a distinct positional component and this has been shown to be caused by a redistribution of blood flow towards the lower lung zones. Platypnea usually occurs in conjunction with orthodeoxia which is defined as a decrease in PaO2 by ≥5% or ≥4 mm Hg when changing from the supine to the upright position. Interestingly, although there is a significant change in PaO2 in orthodeoxia at least one study showed no significant change in DLCO from supine to upright in the same patients. Given the small number of patients (n=5) with orthodeoxia whose DLCO was studied in both the positions it’s unclear whether this applies to all patients with orthodeoxia.
A small number of studies have shown that both the membrane component of the diffusing capacity (DMCO) and the pulmonary capillary blood volume (Vcap) are reduced in HPS. Although the reduced DMCO could be taken as indication of a thickened alveolar-capillary membrane, a finding which is seconded to some extent by the fact that smooth muscle hypertrophy and fibrosis in the small pulmonary arteries have been found in individuals with HPS, a careful analysis in one study indicated that it is more likely an artifact of a reduced pulmonary capillary transit time.
Individuals with HPS usually have a significantly elevated cardiac output. This is due in part to a reduced systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) but may also in part be a response to chronic hypoxia. The elevated cardiac output however, decreases the pulmonary capillary transit time and contributes to hypoxia.
Interestingly, at least one study showed that some individuals with orthodeoxia have an elevated cardiac output while supine and a normal cardiac output while upright. The reason for this finding and whether it is a contributing factor to the orthodeoxia are unclear.
HPS primarily affects the circulatory system so unless a coexisting lung disease is present spirometry and lung volumes are usually normal. Even so a certain number of patients with liver disease will show a restrictive pattern due to ascites, pleural effusion, respiratory muscle weakness and an enlarged liver.
Hypoxemia is usually progressive in hepatopulmonary syndrome and long-term survival rates are poor. HPS used to be considered a contraindication towards liver transplantation but the finding that hypoxia often improves post-transplant has caused this to be re-thought. One study found a 23% 5-year survival rate in patients with HPS that did not receive a liver transplant and a 63% 5-year survival rate among those that did. It is interesting to note that although oxygenation often improves post-transplant, in general DLCO does not. This finding may be related to the length of time that DLCO is studied post-transplant however, since one case study showed that a 7-year post-transplant survivor had an increase in DLCO that was not present 16 months post-transplant.
In many different lung diseases a reduced DLCO is due to a ventilation-perfusion mismatch. This mismatch is often due to a maldistribution of ventilation but hepatopulmonary syndrome is an example of the maldistribution of perfusion. A reduced DLCO seen in the presence of liver disease is frequently a sign of hepatopulmonary syndrome. Transplant centers worldwide have reported that between 5% and 32% of the patients referred for liver transplantation have HPS but the actual frequency among all patients with liver disease is unknown.
Hepatopulmonary syndrome causes systemic and pulmonary vasodilation and through a variety of mechanisms, some of which remain poorly understood, cause hypoxia and a reduced DLCO. The level of hypoxia (and to some extent the reduction in DLCO) are directly related to the severity of HPS. Individuals with severe HPS also tend to have a positional component and show an increase in dyspnea and a decrease in PaO2 when moving from supine to upright and for this reason may need to be evaluated in both positions.
References:
Collisson EA, Nourmand H, Fraiman MH, Cooper CB, Bellamy PE, Farmer DG, Vierling JM, Ghobrial RM, Busuttil RW. Restrospective analysis of the results of liver transplantation for adults with severe hepatopulmonary syndrome. Liver Transpl 2002; 8: 925-931.
Degano B, Mittaine M, Guenard H, Rami J, Garcia G, Kamar N, Bureau C, Peron JM, Rostaing L, Riviere D. Nitric oxide and carbon monoxide transfer in patients with advanced liver cirrhosis. J Appl Physiol 2009; 107: 139-143.
Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Robert KE, Shah VH, Kaplowitz N, Forman L, Wille K, Kawut SM. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroentrology 135(4): 1168-1175.
Gomez FP, Martinez-Palli G, Barbera JA, Roca J, Navasa M, Rodriguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology 2004; 40: 600-666.
Lima BLG, Franca AVC, Pazin-Filho A, Araujo WM, Martinez JAB, Maciel BC, Simoes MV, Terra-Filho J, Martinelli ALC. Frequence, clinical characteristics and respiratory parameters of hepatopulmonary syndrome. Mayo Clin Proc 2004; 79: 42-48.
Rodriguez-Roisin R, Krowka MJ. Is severe arterial hypoxaemia due to hepatic disease an indication for liver transplantation? A new therapeutic approach. Eur Respir J 1994; 7: 839-842.
Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB. ERS Task Force: Pulmonary-hepatic vascular disorders. Eur Respir J 2004; 861-880.
Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome – a liver-induced lung vascular disorder. N Engl J Med 2008: 358: 2378-2387.
Scarlata S, Conte ME, Cesari M, Gentilucci UV, Miglioresi L, Pedone C, Picardi A, Ricci GL, Incalzi RA. Gas exchanges and pulmonary vascular abnormalities at different stages in chronic liver disease. Liver International 2011; 31(4): 525-533.
Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology 2005; 41: 1122-1129.
West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol 1962; 17(6): 893-898.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License

Just about the best summary of my HPS! Thanks