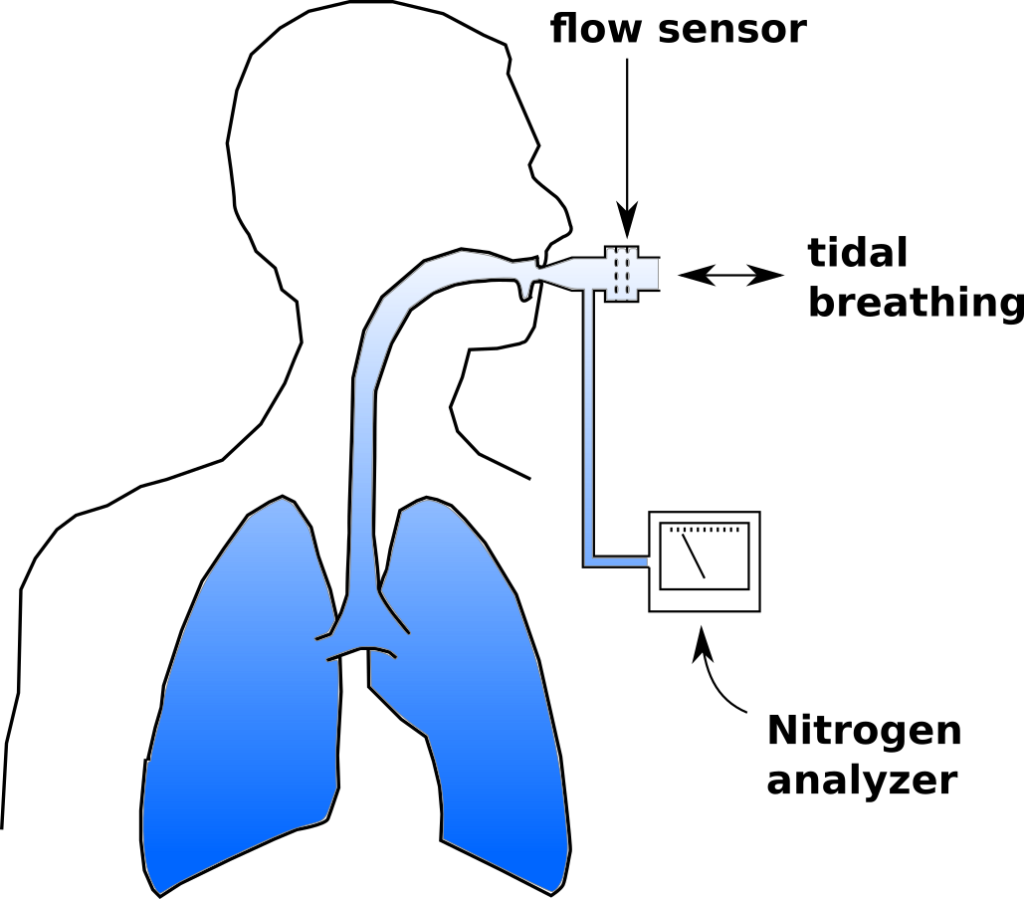
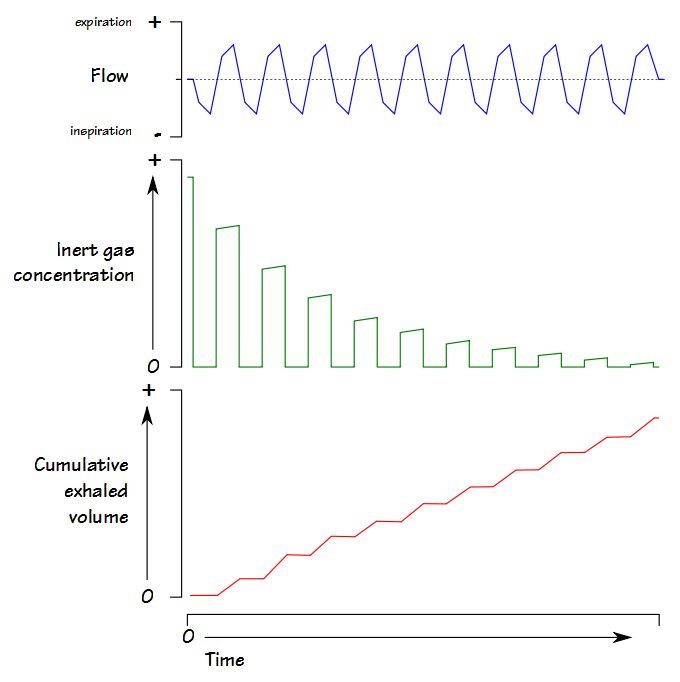
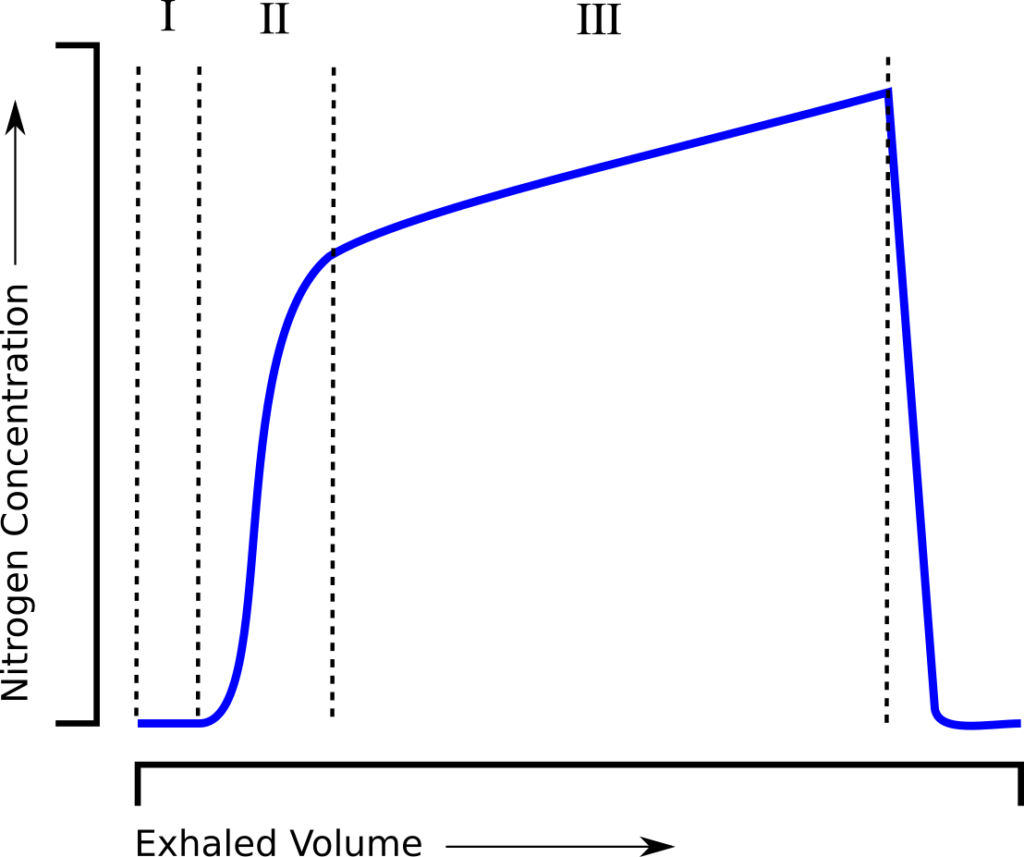
The Lung Clearance Index (LCI) was first described in 1952 by Margaret Becklake, and is defined as the number of lung volume turnovers required to reduce the concentration of a tracer gas by a factor of 40. LCI is calculated as the cumulative exhaled volume (CEV) during the washout divided by the functional residual capacity (FRC).
Clinically LCI has been used most often in individuals with cystic fibrosis and this is because the LCI has been repeatably shown to be sensitive to changes in airway status that are not reflected in the FEV1. LCI has shown similar results in patients with primary ciliary dyskinesia. As expected LCI has also been tested on patients with COPD, bronchiectasis and asthma although these patients tend to show a better correlation between FEV1 and LCI.
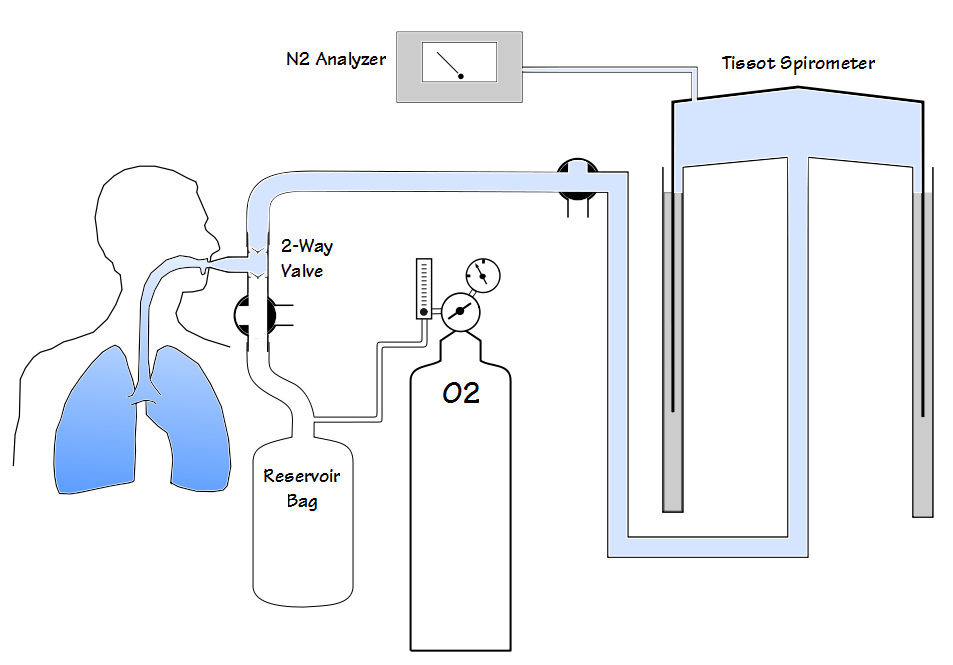
LCI has been performed using a wide variety of tracer gases including helium, methane, argon, nitrogen and sulfur hexaflouride (SF6). The commercial systems that are currently available use either N2 or SF6. N2 washout LCI has recently received a great deal of criticism and some of these criticisms seem to apply to N2 washout lung volumes as well.
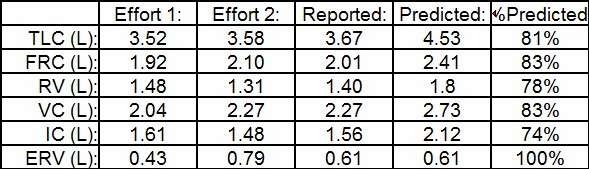
Most specifically, a number of studies have noted that the N2 washout FRC is routinely higher than the SF6 FRC and plethysmographic FRC. In addition, the N2 washout LCI tends to be significantly higher than the SF6 LCI and this difference increases as LCI increases.
As examples in a study of patients with COPD the N2 washout FRC averaged 14% higher than the plethysmographic FRC. In other studies of normal subjects the N2 washout FRC was on average 0.20 to 0.21 L higher than plethysmographic FRC. Finally, a study that performed N2 and SF6 washouts simultaneously on CF patients and healthy controls showed the N2 washout LCI to be on average 7.93% higher than SF6 in the healthy controls and 29.13% higher than SF6 in the CF patients. In the same study N2 washout FRC was 12.66% higher than SF6 FRC in the healthy controls and 30.09% higher than SF6 FRC in CF patients.
So why is there such a discrepancy?
Continue reading