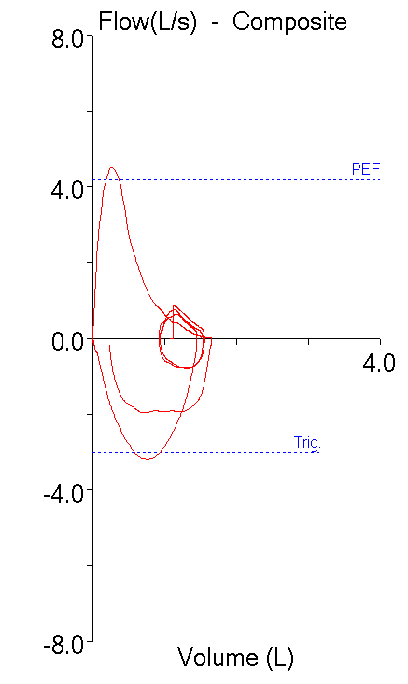
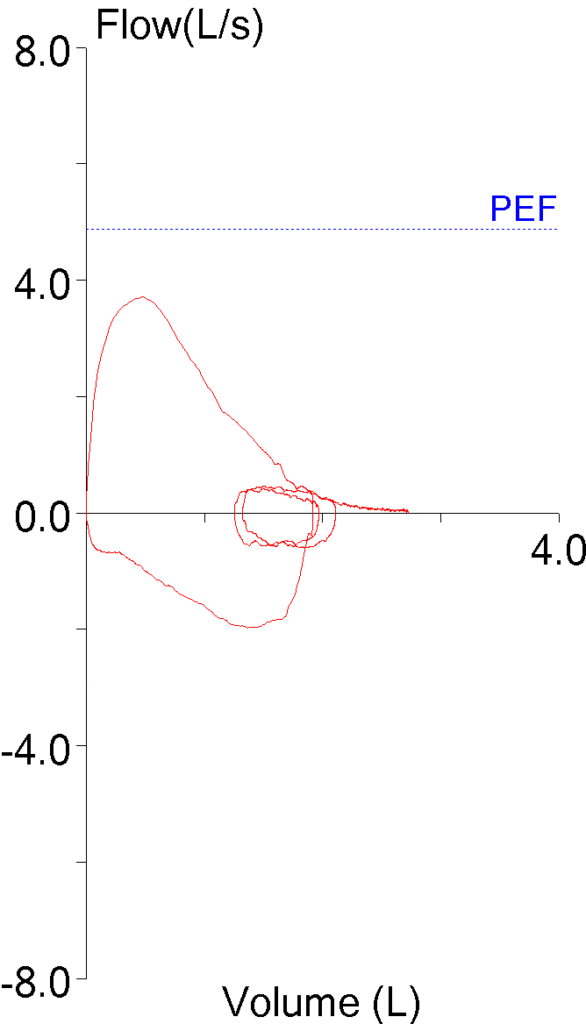
The 2019 ATS/ERS Spirometry Standards were recently released. The standards are open-access and can be downloaded without charge from the October 15th issue of the American Journal of Respiratory and Critical Care Medicine. Supplements are available from the same web page.
The 2019 Spirometry Standards have been extensively re-organized with numerous updates. Notably, a number of sections that were previously discussed in the 2005 General Considerations for Lung Function Testing have been updated and included in the 2019 Spirometry Standards. Also notably, a number of stand-alone spirometry tests, including the Flow-Volume Loop, PEF and MVV are not included in the 2019 Standards.
An overview of changes and updates from the 2005 Spirometry Standards are detailed within the 2019 Spirometry Standards (page e71, column 1, paragraph 2) and in the Data Supplement (pages E2-E3). In more detail these include:
◆ The list of indications for spirometry (page e73, table 1) was updated primarily with changes in language.
- “To measure the effect of disease on pulmonary function” was updated to “To measure the physiological effect of disease or disorder”
- “To describe the course of diseases that affect lung function” was updated to “To monitor disease progression”
- “To monitor people exposed to injurious agents” was updated to “To monitor people for adverse effects of exposure to injurious agents”
◆ Items added to indications:
- “Research and clinical trials”
- “Preemployment and lung health monitoring for at-risk occupations”
◆ Contraindications were previously mentioned in the 2005 General Considerations rather than the 2005 Spirometry Standards and these have been extensively updated and expanded. Although the list of contraindications (page e74, table 2) is fairly inclusive (and should be reviewed by all concerned) there were items mentioned in the body of text that were not in the table:
- “Spirometry should be discontinued if the patient experiences pain during the maneuver.”
- “…because spirometry requires the active participation of the patient, inability to understand directions or unwillingness to follow the directions of the operator will usually lead to submaximal test results.”
◆ Notably, abdominal aortic aneurysm (AAA) was not included as a contraindication in the 2019 standards. (page e72, column 3, paragraph 1)
Continue reading