Recently I was trying to explain the effect of altitude on blood oxygenation to somebody with IPF. They had observed that their oxygen saturation was fairly normal at sea level but that they needed to use their supplemental O2 when they went up to 2000 feet and didn’t understand why such a low altitude made that much of a difference. I don’t think I did very well with the explanation since at the time I was limited to only text and I much prefer pulling out diagrams and waving my hands in the air.
The place to start is with the alveolar air equation.
Where:
PAO2 = partial pressure of O2 in the alveoli
FiO2 = fractional concentration of O2
PB = barometric pressure
PH20 = partial pressure of water vapor in the alveoli
PaCO2 = partial pressure of CO2 in the arteries
RER = respiratory exchange ratio (VCO2/VO2)
When normal values are plugged into the alveolar air equation it looks like this:
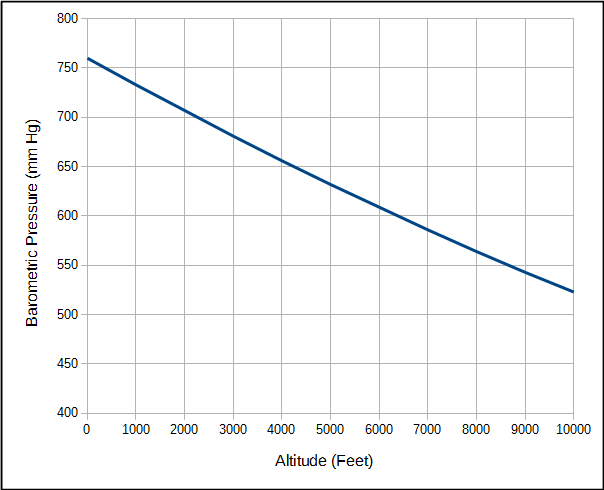
An important point is that when air is inhaled into the lung oxygen is diluted by the water vapor and carbon dioxide that are already there. The partial pressure of oxygen will vary with atmospheric pressure but the partial pressure of water vapor and carbon dioxide are relatively fixed values. Atmospheric pressure of course decreases with altitude.
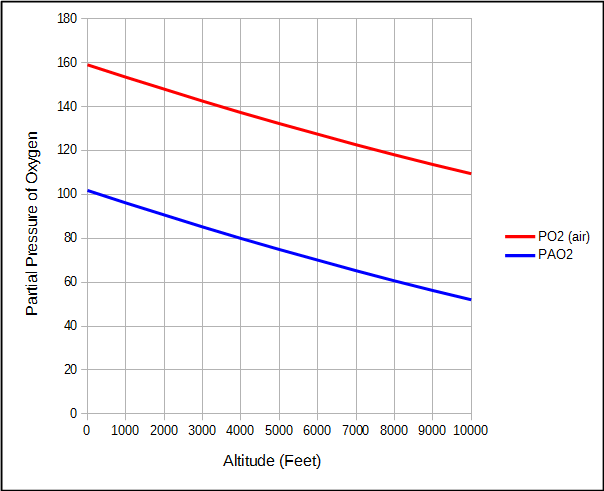
For the same reason, the partial pressure of oxygen in air and the alveoli also decreases as altitude increases.
But because water vapor and carbon dioxide are relatively constant, the partial pressure of alveolar oxygen decreases faster than the partial pressure of O2 in air.
This means that although the atmospheric pressure at 2000 feet is 7% less than at sea level, alveolar PO2 is 11% less.
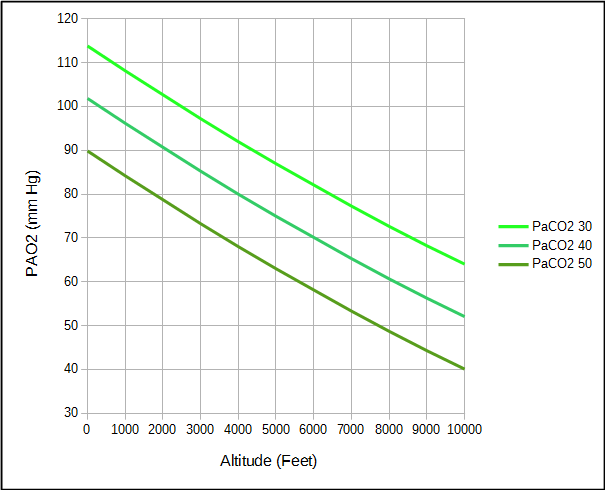
However, not every individual’s arterial PCO2 and respiratory exchange ratio are “normal” and differences can either increase or decrease the alveolar PO2. For example, an elevated PaCO2 decreases PAO2 while a reduced PaCO2 increases PAO2.
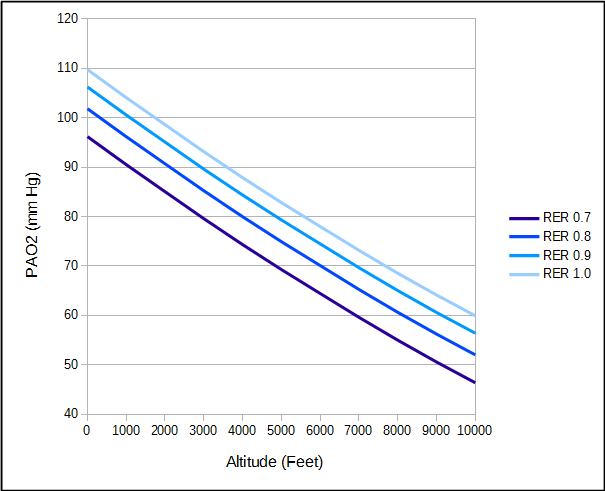
The respiratory exchange ratio is affected both by diet and activity. A high protein diet tends to decrease RER while a high carbohydrate diet or activity tends to increase RER.
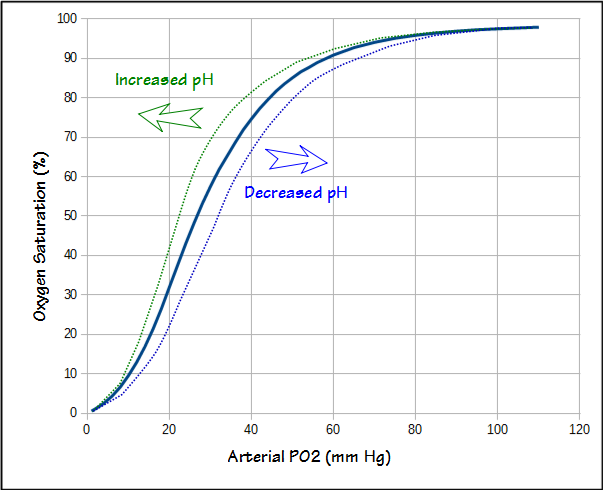
The alveolar air equation only addresses the oxygen concentration in the alveoli. To be of any use oxygen needs to get into the bloodstream. Although diffusing capacity is a measurement of gas exchange efficiency it is not possible to take the DLCO and PAO2, and then predict arterial PO2 (PaO2) with any accuracy. Nor is it possible to measure the arterial oxygen saturation (SaO2) with a pulse oximeter and then calculate PaO2. This is because pulse oximeter accuracy is only +/- 3% and blood pH also affects the oxygen dissociation curve.
PaO2 must therefore be measured from an arterial blood sample. When this is done you can calculate the difference between alveolar and arterial PO2 (known alternately as the A-a gradient or PAaO2). The A-a gradient rises with age and at sea level the normal value is approximately:
Results from several studies however, indicate that the A-a gradient decreases with altitude. The decrease is small, only 0.02 mm Hg per mm Hg decrease in atmospheric pressure which is roughly 1 mm Hg per 2000 foot increase in altitude.
Using these formulas and ostensibly normal values for a 60 year old, PAO2, PaO2 and SaO2 would be:
| Altitude (ft): | PAO2: | A-a Gradient: | PaO2: | SaO2: |
| 0 | 101.8 | 19.0 | 82.8 | 96.0% |
| 2000 | 90.7 | 18.0 | 72.7 | 94.6% |
| 4000 | 80.0 | 17.0 | 63.0 | 89.7% |
| 6000 | 70.1 | 16.0 | 54.1 | 87.8% |
| 8000 | 60.7 | 15.0 | 45.7 | 81.5% |
| 10000 | 52.1 | 14.0 | 38.1 | 72.0% |
But the oxygen saturation values at the higher altitudes seem excessively low and this is because one of the adaptations to altitude is an increase in ventilation. This causes PaCO2 to decrease and pH to increase. The decrease in PaCO2 increases PAO2. The increase in pH shifts the oxygen dissociation curve leftwards which means that the SaO2 for a given PaO2 also increases. At 10,000 feet with a PaCO2 of 30 and pH of 7.50 (much more extreme values such as pH of 7.60 and PaCO2 of 20 have been measured in mountain climbers at extreme altitudes), PAO2 would be 64, PaO2 would be 50, and SaO2 would be 92%.
Even a modest change in PaCO2 to 35 without any corresponding change in pH makes a significant change in oxygenation.
| Altitude (ft): | PAO2: | A-a Gradient: | PaO2: | SaO2: |
| 0 | 107.8 | 19.0 | 88.8 | 98.0% |
| 2000 | 96.7 | 18.0 | 78.7 | 95.6% |
| 4000 | 86.0 | 17.0 | 69.0 | 93.8% |
| 6000 | 76.1 | 16.0 | 60.1 | 90.9% |
| 8000 | 66.7 | 15.0 | 51.7 | 86.3% |
| 10000 | 58.0 | 14.0 | 44.0 | 79.6% |
Airplanes that travel at 30,000 to 40,000 feet are usually pressurized to an equivalent altitude of 6000 to 8000 feet and the lower limit of normal for the SaO2 of airplane travelers is usually considered to be between 89% and 91%.
Everybody’s arterial oxygen decreases as altitude increases. The decrease in the availability of oxygen with increasing altitude is due to the decrease in atmospheric pressure but is also influenced by the relatively constant amounts of water vapor and carbon dioxide in the lung. Alveolar O2 therefore decreases faster than would be expected for a simple change in atmospheric pressure. Increased ventilation is a compensatory mechanism that causes changes in PaCO2 and pH and makes oxygen more available. Lung disease tends to decrease the efficiency of gas exchange which increases the A-a gradient and may also limit an individual’s ability to increase ventilation and compensate for hypoxia. For these reasons sometimes only modest changes in altitude are enough to make a significant change in SaO2.
References:
Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med 1999; 160: 1525-1531
Ruppel GL. Manual of pulmonary function testing, 8th edition, 2003.
Vincent J, Hellot MF, Vargas E, Gautier H, Pasquis P, Lefrancois R. Pulmonary gas exchange, diffusing capacity in natives and newcomers at high altitude. Repir Physiol 1978; 34: 219-231.
Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol 1986; 61: 260-270.

PFT Blog by Richard Johnston is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License


Hi
A very useful and succinct piece of writing. I am a newly diagnosed IPF patient ,not yet on O2 but prob not far off. I am on a driving holiday in France and have spent a few days in the Auvergne area at altitude 3000 -4000 feet and noticed how my O2%sats have fallen and chest has ‘tightened’. I’m now back at near sea level for the rest of our holidayand feeling much better. Do you mind if I post a link to your blog on the pulmonary fibrosis uk Facebook page please as a warning to other IPS sufferers
Alan –
I’m glad you found it informative. Of course, feel free to post a link (I can’t think of a single blog writer that doesn’t want more readers!) but I have to admit I’d rather it was thought of as an explanation than as a warning.
– Richard
hey i found it informative but can u tell me about a direct or simplified relation so that we can calculate the saturation of O2 for any given value of barometric pressure of O2 (with respect to altitude).
Karan –
There is no simple relationship between altitude and oxygen saturation. Saturation doesn’t just depend on the partial pressure of oxygen in the air that you breathe (which of course is dependent on altitude) but also to the amount of carbon dioxide in your blood, the pH of your blood and your respiratory exchange ratio. These can’t be predicted, only measured, and what they are for any given person will depend on how well they adapt/respond to altitude.
– Richard
I suffer from idiopathic pulmonary fibrosis, polycythaemia rubra Vera, and more recently pulmonary hypertension. At my last pulmonary function test I had FVC 49% and TLCO 21%. I regularly holiday in the summer in the ALPS and we normally stay at altitudes of 5000 ft and walk at 8000 ft. I am on oxygen for exercise 2lpm and I always use it when walking, and also when ballroom dancing. Am I adding to my declining breathlessness by walking at higher altitudes?
I have survived 10 years post IPF diagnosis.
Malcolm –
I congratulate you on staying active since that is probably the best thing you could be doing for yourself. The body’s response to low oxygen levels in the blood is usually to increase cardiac output (usually by increasing heart rate) and to increase ventilation (increased tidal volume and respiratory rate), but whether this actually causes harm is unclear and I would have to say it depends a lot on the health status and problems of each person. I’ve discussed what is the lowest safe level of oxygen (SaO2) for patients with doctors for years and the one thing I’ve found is that there is no consensus of opinion on this issue. Some doctors get nervous when SaO2 drops below 90% and others say it’s okay to let it drop below 80%. The decision in my lab is to stop an exercise test (CPET or 6-minute walk) whenever SaO2 drops to 84% or below but that is somewhat arbitrary (it’s based on the fact that 84% is on the steep portion of the oxygen dissociation curve and small decreases in arterial PO2 at that point cause big changes in SaO2). 2 LPM of O2 may be sufficient to keep your SaO2 in a safe area for you even at altitude but I’d say you shouldn’t ignore symptoms like muscle cramps, chest pain or severe shortness of breath. Otherwise, if you’re feeling okay it’s hard for me to say you shouldn’t be doing what your’re doing.
– Richard
Absolutely excellent!
Most informative 15 minutes i’ve spent in a very very long time.
Thank you!
hello,
i am a medical student who knows nothing however i am learning about A-a gradients in class and there seems to be an error. There should not be a change in A-a gradient with high altitude.
K –
The A-a gradient changes if for no other reason than PACO2 changes due to changes in ventilation with altitude.
– Richard