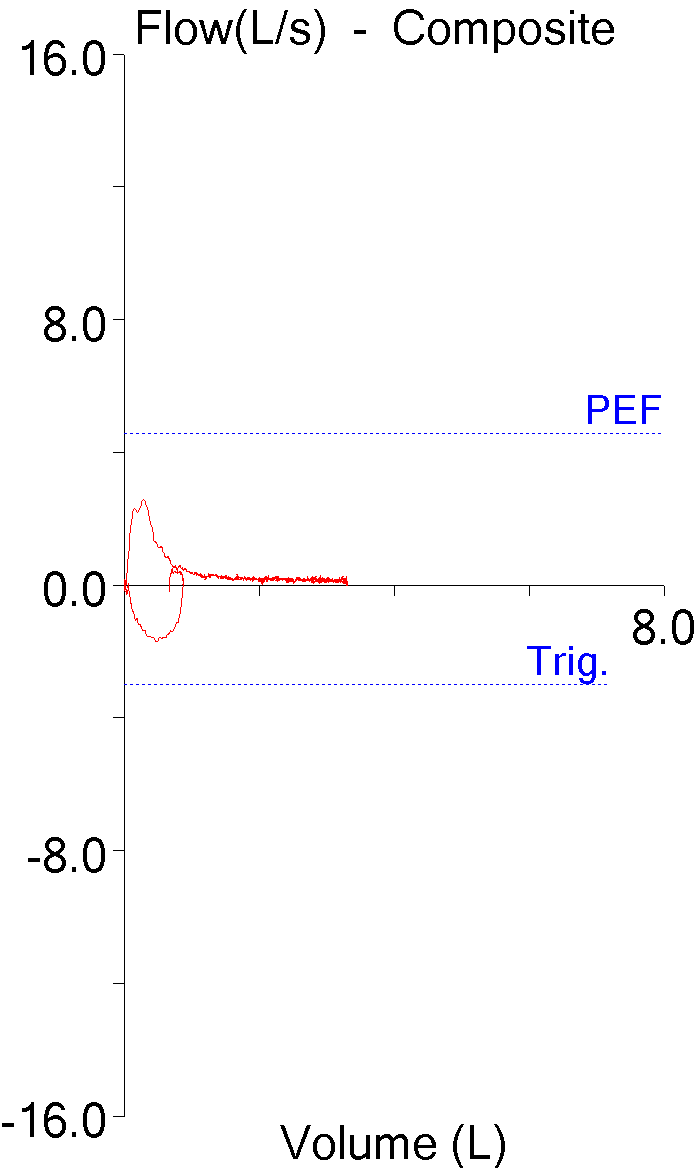
The patients whose reports I review have always been very accommodating. An issue of one kind or another catches my attention and before I know it I find several more reports that are similarly involved. Thanks to our patients I’ve had a number of reports come across my desk recently that showed a combination of restrictive and obstructive defects. This particular one may not be the best possible example but it seems to illustrate several points fairly well.
| Observed: | %Predicted: | Predicted: | |
| FVC (L): | 1.12 | 40% | 2.80 |
| FEV1 (L): | 0.75 | 35% | 2.16 |
| FEV1/FVC (%): | 67 | 86% | 78 |
| TLC (L): | 1.92 | 42% | 4.54 |
| FRC (L): | 1.18 | 48% | 2.47 |
| RV (L): | 0.76 | 44% | 1.73 |
| RV/TLC (%): | 40 | 104% | 38 |
Interpreting results like this as combined (or mixed) defects using the ATS/ERS algorithm seems relatively straightforward.
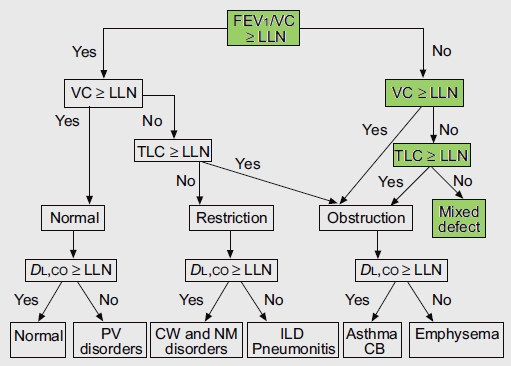
From Brusasco V, Crapo R, Viegi G. ATS/ERS Task Force: Standardisation of pulmonary function testing. Interpretive strategies for lung function tests. Eur Respir J 2005; 26, page 956
The algorithm starts by using the FEV1/FVC ratio to determine whether obstruction is present and only then considers whether or not the FVC and TLC are normal. It occurred to me however, that this assumes that the normal range of the FEV1/FVC ratio is preserved when TLC decreases below normal. Given the markedly different causes of restrictive lung disease it would seem that saying that the FEV1/FVC ratio should remain within the normal range over a relatively broad range of lung capacities (and without necessarily knowing the cause for any reduction) seems a bit far-fetched. Interestingly enough however, it actually turns out to be reasonably true.