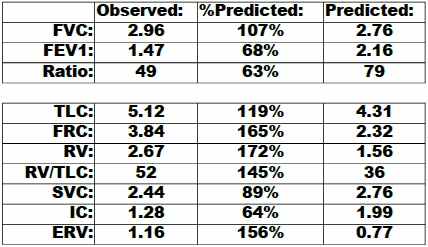
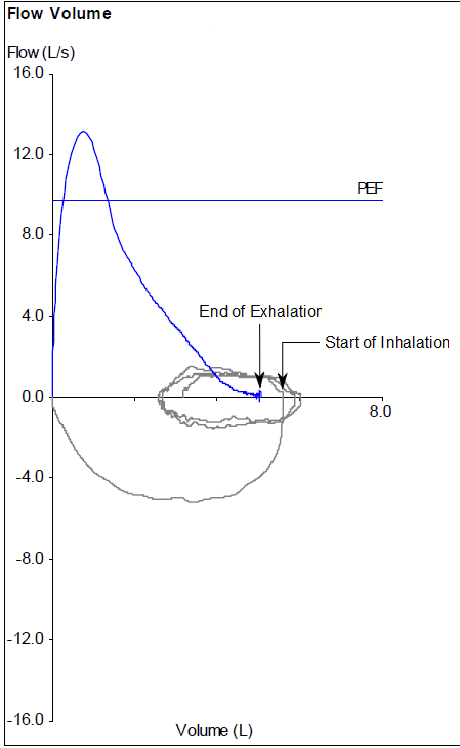
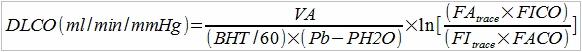The PFT Lab I am associated with performs cardiopulmonary exercise tests for pre-op cardiothoracic surgery patients with lung cancer. Surgeons have to make a decision to operate or not based at least in part on the amount of function they think will remain after a lung resection and this depends on which and how many lobes are involved. This calculation is done by taking a percentage based on the amount of lung tissue that will be lost which is weighted using ventilation-perfusion scan results off the baseline DLCO and vital capacity. When the result is inconclusive or there are other medical factors that can affect a patient’s prognosis the results from a CPET can help with the surgeon’s decision.
The criteria that has been most often cited as an indicator of acceptable surgical risk is a peak VO2 greater than 15 ml/kg/min. About ten years ago we encountered a patient with a significantly low body weight. His max VO2 was about 16 ml/kg/min which appeared to make him a good candidate for surgery but his actual maximum oxygen consumption in LPM was 45% of predicted. This discrepancy was due to the fact that his BMI was about 14. We re-calculated what his peak VO2 would have been if his body weight had been normal and that was less than 10 ml/kg/min. For this reason our recommendation at that time was that the patient was a poor candidate for surgery. Continue reading